Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (10): 114-118.doi: 10.19964/j.issn.1006-4990.2021-0007
• Chemical Analysis & Monitoring • Previous Articles Next Articles
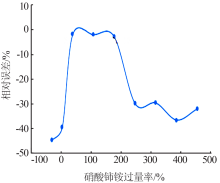
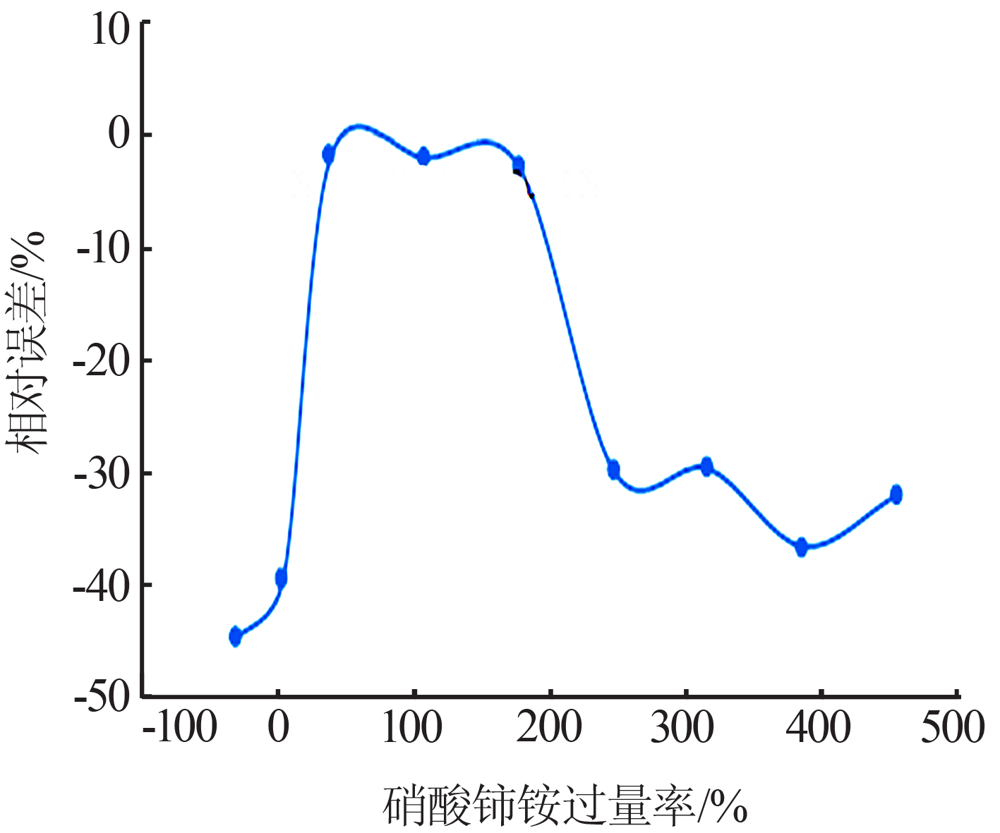
Study on a new method for determination of hypophosphorous acid
Zheng Na,Zhou Junhong( ),Chen Hangxin,Chen Shengyue,Wan Zongjing,Sheng Yu
),Chen Hangxin,Chen Shengyue,Wan Zongjing,Sheng Yu
- School of Chemistry and Chemical Engineering,Qiannan Normal University for Nationalities,Duyun 558000,China