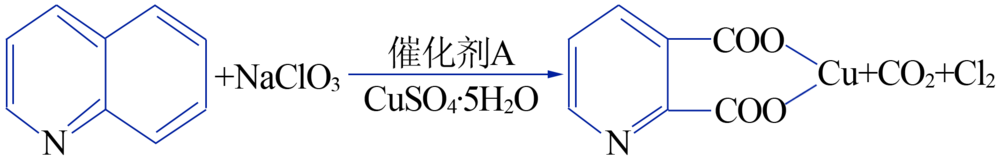
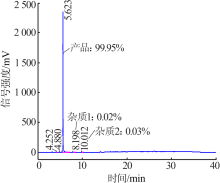
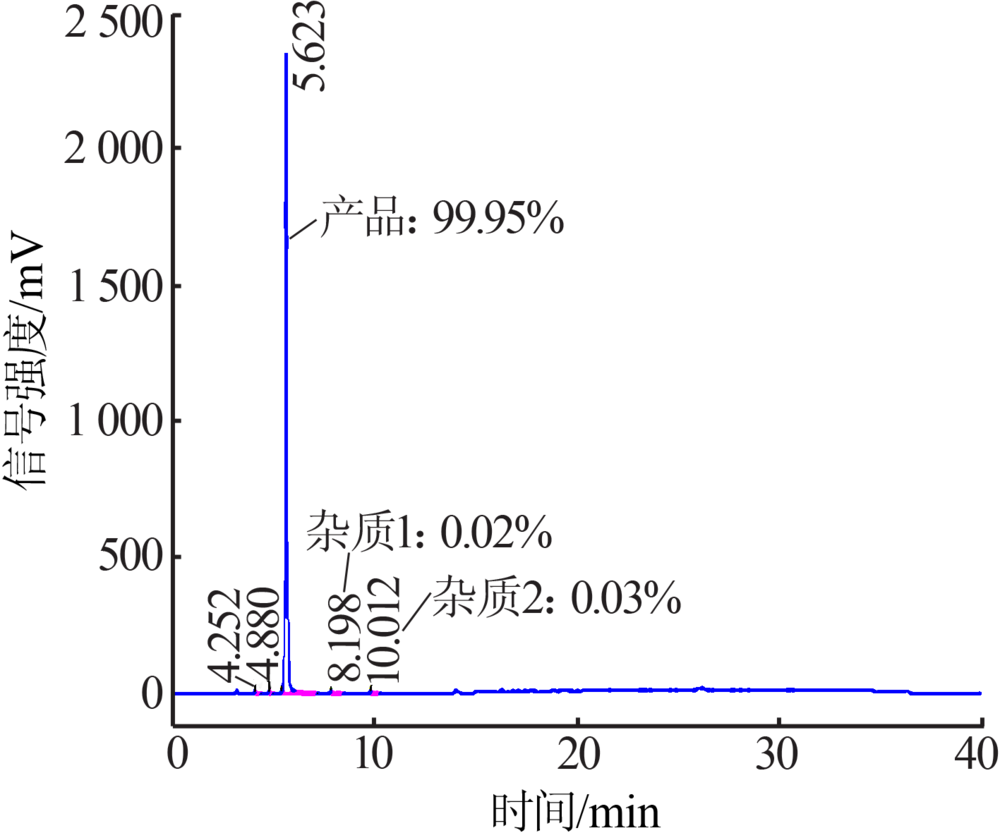
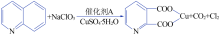| [1] |
TANG Kaijing, LIU Chuanbei, LI Yingding, JIANG Yong, WU Junnan, ZHANG Tao.
Research on preparation and mechanism of superhydrophobic phosphogypsum products
[J]. Inorganic Chemicals Industry, 2025, 57(1): 97-102.
|
| [2] |
LIU Xiaowen, LI Jun, ZHOU Zhaoan, MAO Anzhang, ZHOU Aiqing.
Study on response surface methodology optimization of PAC for deep purification of fluorine ion in high concentration sodium sulfate solution
[J]. Inorganic Chemicals Industry, 2024, 56(6): 67-72.
|
| [3] |
SHEN Haiyan, LI Fangqin, REN Jianxing, WU Jiang, GUAN Zhenzhen, PAN Weiguo.
Research progress on chemical absorption method for capturing carbon dioxide
[J]. Inorganic Chemicals Industry, 2024, 56(5): 11-19.
|
| [4] |
LU Yunkun, TANG Xianyou, YIN Hang, ZHANG Yanan, ZHANG Shaojie.
Study on surface packaging and leakage prevention of high temperature molten salt/ceramic composite phase change thermal storage materials
[J]. Inorganic Chemicals Industry, 2024, 56(2): 80-85.
|
| [5] |
LIU Yichang, XIE Zhipeng, LIU Yunfeng, ZHANG Da, LIANG Feng.
Study on impedance matching strategy of enhancing microwave absorption performance of nitrogen-doped single-walled carbon nanohorns
[J]. Inorganic Chemicals Industry, 2024, 56(12): 29-34.
|
| [6] |
LI Hongyuan, ZHANG Jianhua.
Study on removal process of total organic carbon from industrial waste salts by pyrolysis
[J]. Inorganic Chemicals Industry, 2024, 56(10): 95-102.
|
| [7] |
WEI Tianshun, JI Lijun, SHENG Yong, CHEN Kui, WU Yanyang, WU Bin.
Study on fractional crystallization process of ammonium sulfate and sodium sulfate in high salt wastewater
[J]. Inorganic Chemicals Industry, 2024, 56(1): 102-106.
|
| [8] |
ZHOU Zhaoan, LI Jun, LIU Xiaowen, ZHOU Aiqing, MAO Anzhang.
Study on carbonization and purification process of high COD industrial waste salt
[J]. Inorganic Chemicals Industry, 2023, 55(9): 100-105.
|
| [9] |
CHEN Tao, GAO Huimin.
Study on preparation of phosphorus trichloride by microreaction
[J]. Inorganic Chemicals Industry, 2023, 55(4): 72-75.
|
| [10] |
DUAN Rui, JIA Zhaohang, XU Maolan, MA Tianyue, PENG Wencai, ZHANG Jianshu.
Study on thermal chlorination and mechanism of chlorodimethylsilane and trichlorosilane
[J]. Inorganic Chemicals Industry, 2023, 55(12): 82-87.
|
| [11] |
PEI Xiu,LI Yaming.
Study on preparation of covalent organic framework materials and their adsorption properties for dyes
[J]. Inorganic Chemicals Industry, 2023, 55(1): 106-111.
|
| [12] |
FANG Weicheng,CHENG Xingxing,SUN Changrong.
Optimization of preparation of sludge/fly ash composite ceramsite filler materials by response surface methodology
[J]. Inorganic Chemicals Industry, 2022, 54(9): 119-125.
|
| [13] |
RONG Xilin,CUI Bao,HUANG Qiumei,CUI Meijia,CHENG Hao,FENG Jun,HUANG Wenyi.
Study on preparation of bagasse cellulose based carbon aerogels and their adsorption properties
[J]. Inorganic Chemicals Industry, 2022, 54(9): 126-135.
|
| [14] |
WANG Bingjian,QI Daozheng,CHEN Deng.
Degradation processes and mechanism of tricalcium aluminate in different sulfate environment
[J]. Inorganic Chemicals Industry, 2022, 54(8): 119-124.
|
| [15] |
ZHENG Sanqiang,LI Xingbin,LUO Xingguo,WEI Chang,HUANG Xing,DENG Zhigan,LI Minting.
Production and control of water insoluble substance in sodium sulfate produced by NaCl-Na2SO4 co?production
[J]. Inorganic Chemicals Industry, 2022, 54(8): 90-95.
|