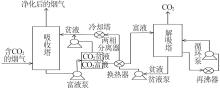
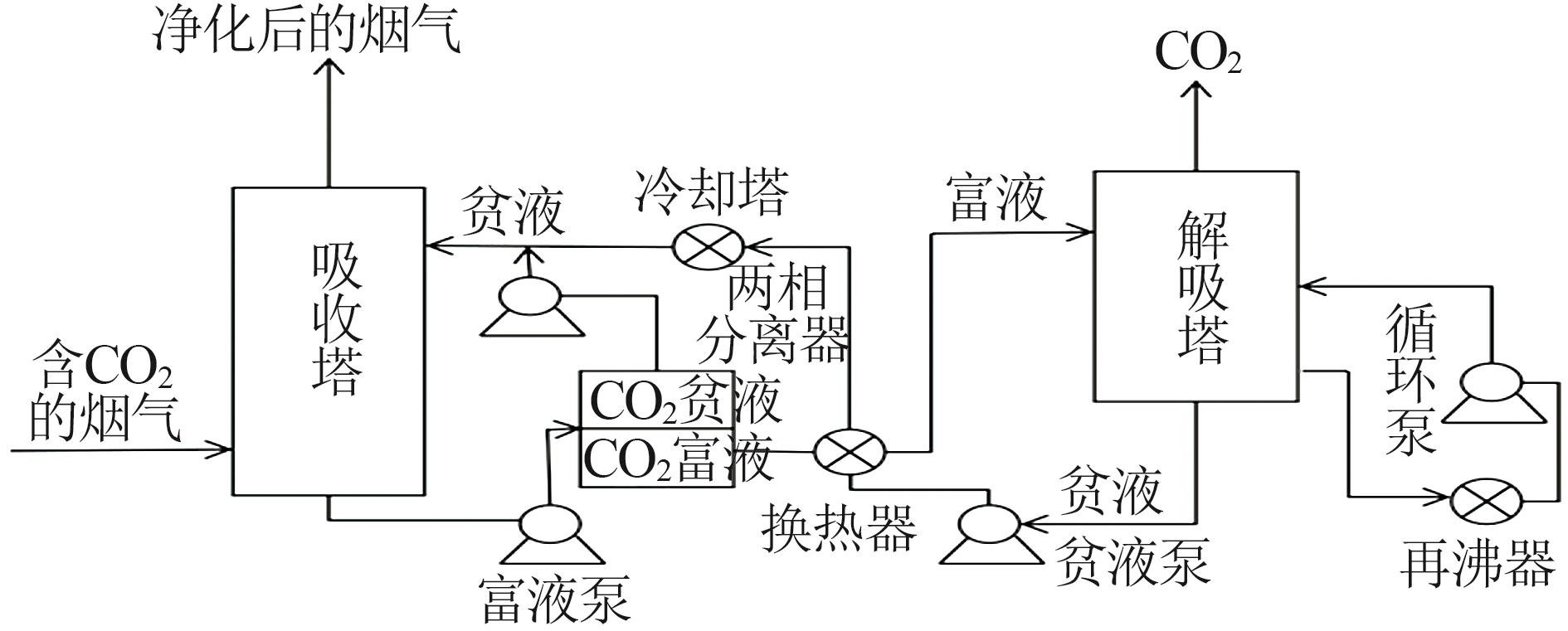
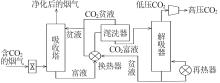
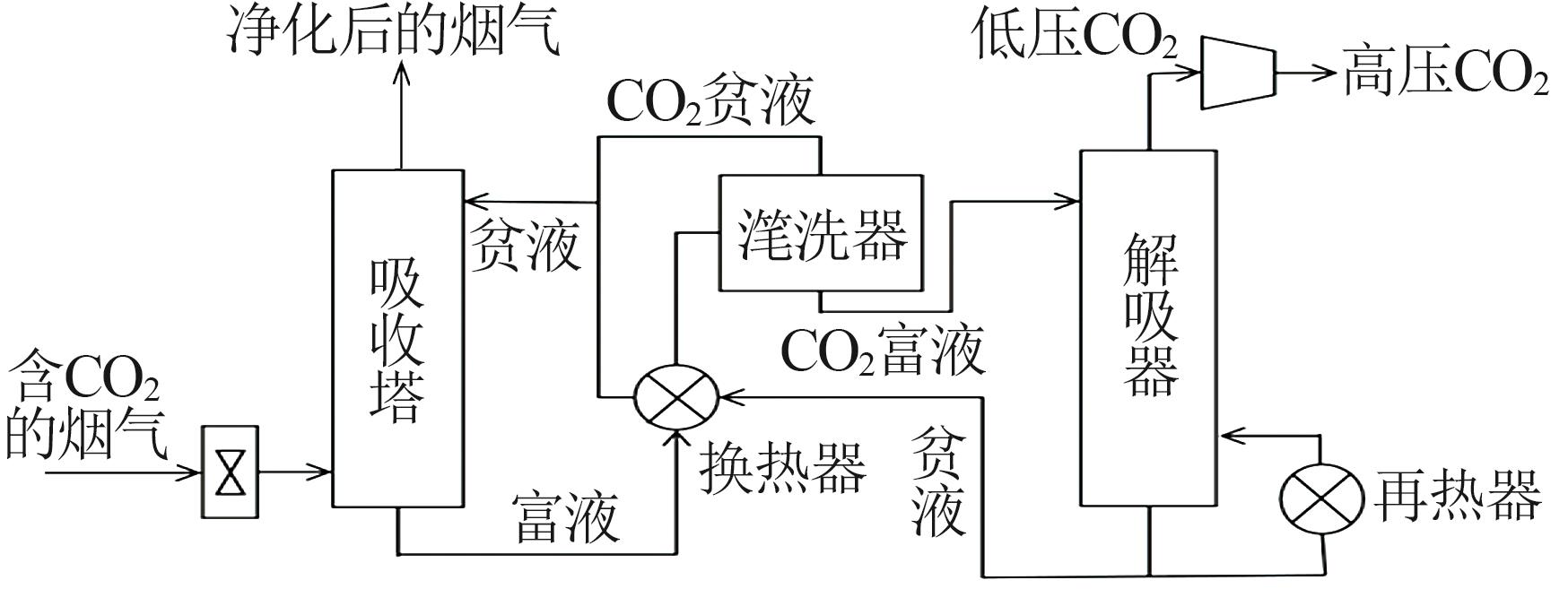
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (5): 11-19.doi: 10.19964/j.issn.1006-4990.2023-0446
• Reviews and Special Topics • Previous Articles Next Articles
Research progress on chemical absorption method for capturing carbon dioxide
SHEN Haiyan1( ), LI Fangqin1,2(
), LI Fangqin1,2( ), REN Jianxing1, WU Jiang1, GUAN Zhenzhen1, PAN Weiguo1,2
), REN Jianxing1, WU Jiang1, GUAN Zhenzhen1, PAN Weiguo1,2
- 1.College of Energy and Mechanical Engineering, Shanghai University of Electric Power, Shanghai 201306, China
2.Shanghai Non-Carbon Energy Conversion and Utilization Institute, Shanghai 200240, China
-
Received:2023-09-07Online:2024-05-10Published:2024-05-15 -
Contact:LI Fangqin E-mail:1825504173@qq.com;lifangqin@shiep.edu.cn
CLC Number:
Cite this article
SHEN Haiyan, LI Fangqin, REN Jianxing, WU Jiang, GUAN Zhenzhen, PAN Weiguo. Research progress on chemical absorption method for capturing carbon dioxide[J]. Inorganic Chemicals Industry, 2024, 56(5): 11-19.
share this article
Table 2
Comparison of properties of different absorbers"
| 吸收剂类型 | 优点 | 缺点 |
|---|---|---|
| 有机胺溶液吸收剂 | 成本较低、捕集能力强、反应稳定和技术成熟 | 混合胺吸收剂仍需深入研究,进一步降低再生能耗,减少设备腐蚀,才能最终实现大规模工业化应用 |
| 氨水溶液吸收剂 | 与传统的MEA溶液相比,效率更高,成本更低,能协同脱除多种酸性气体 | 氨逃逸现象会造成环境污染、仪器堵塞的问题,严重限制了氨法的应用 |
| 离子液体吸收剂 | CO2溶解度高、选择性好、稳定性好、挥发性低、腐蚀性低 | 吸收容量低、黏度高、成本高昂,需要通过开发简单的合成方法来降低成本 |
| 新型相变吸收剂 | 与传统的MEA溶液相比,新型相变吸收剂的吸收容量和循环容量更高,再生能耗更低,可降至1 610~2 870 kJ/kg | 目前对于新型相变吸收剂的研究还不够成熟,难以应用于工业,对于新型相变溶液的挥发和降解特性研究还不足 |
| 1 | ZHANG Zhien, WANG Tao, BLUNT M J,et al.Advances in carbon capture,utilization and storage[J].Applied Energy,2020,278:115627. |
| 2 | XU Shengqing, DAI Shuiping.CCUS as a second-best choice for China′s carbon neutrality:An institutional analysis[J].Climate Policy,2021,21(7):927-938. |
| 3 | XIN Kun, HASHISH M, ROGHAIR I,et al.Process simulation and economic analysis of pre-combustion CO2 capture with deep eutectic solvents[J].Frontiers in Energy Research,2020,8:573267. |
| 4 | DU Yongbo, ZHAO Nan, WANG Junxiong,et al.Effect of CO2 on coal devolatilization in oxy-fuel combustion:Exploring the influence of temperature and inherent alkali/alkaline earth metal[J].International Journal of Greenhouse Gas Control,2020,96:103004. |
| 5 | TOFTEGAARD M B, BRIX J, JENSEN P A,et al.Oxy-fuel combustion of solid fuels[J].Progress in Energy and Combustion Science,2010,36(5):581-625. |
| 6 | HOU S S, CHIANG C Y, LIN Tahui.Oxy-fuel combustion characteristics of pulverized coal under O2/recirculated flue gas atmospheres[J].Applied Sciences,2020,10(4):1362. |
| 7 | COZIER M.Japan’s role in carbon capture and storage[J].Greenhouse Gases:Science and Technology,2018,8(2):215-217. |
| 8 | LIANG Zhiwu, FU Kaiyun, IDEM R,et al.Review on current advances,future challenges and consideration issues for post-combustion CO2 capture using amine-based absorbents[J].Chinese Journal of Chemical Engineering,2016,24(2):278-288. |
| 9 | 王建行,赵颖颖,李佳慧,等.二氧化碳的捕集、固定与利用的研究进展[J].无机盐工业,2020,52(4):12-17. |
| WANG Jianhang, ZHAO Yingying, LI Jiahui,et al.Research progress of carbon dioxide capture,fixation and utilization[J].Inorganic Chemicals Industry,2020,52(4):12-17. | |
| 10 | 李耀东,李振林,王辉,等.燃烧后化学吸收法脱碳节能工艺研究进展[J].现代化工,2023,43(4):60-65. |
| LI Yaodong, LI Zhenlin, WANG Hui,et al.Research progress on decarburization and energy-saving processes by post-combustion chemical absorption[J].Modern Chemical Industry,2023,43(4):60-65. | |
| 11 | 刘飞,关键,祁志福,等.燃煤电厂碳捕集、利用与封存技术路线选择[J].华中科技大学学报(自然科学版),2022,50(7):1-13. |
| LIU Fei, GUAN Jian, QI Zhifu,et al.Technology route selection for carbon capture utilization and storage in coal-fired power plants[J].Journal of Huazhong University of Science and Technology(Natural Science Edition),2022,50(7):1-13. | |
| 12 | 赵东亚,王家凤,田群宏,等.MEA法碳捕集工艺再生塔能耗优化[J].中国石油大学学报(自然科学版),2021,45(2):181-186. |
| ZHAO Dongya, WANG Jiafeng, TIAN Qunhong,et al.Energy consumption optimization of regeneration tower for MEA CO2 capture process[J].Journal of China University of Petroleum(Edition of Natural Science),2021,45(2):181-186. | |
| 13 | FERRARA G, LANZINI A, LEONE P,et al.Exergetic and exergoeconomic analysis of post-combustion CO2 capture using MEA-solvent chemical absorption[J].Energy,2017,130:113-128. |
| 14 | 张亚萍.乙醇胺捕集燃煤烟气二氧化碳工艺模拟[J].无机盐工业,2022,54(8):96-100. |
| ZHANG Yaping.Process simulation of carbon dioxide capture from coal-firing flue gas by ethanolamine[J].Inorganic Chemicals Industry,2022,54(8):96-100. | |
| 15 | 彭擎东.DIPA法对天然气中CO2组分的回收研究[J].化工管理,2019(30):85-86. |
| 16 | 陆诗建,李清方,张建.醇胺溶液吸收二氧化碳方法及反应原理概述[J].科技创新导报,2009,6(13):4-5,7. |
| LU Shijian, LI Qingfang, ZHANG Jian.Overview of CO2 absorption with organic amines solution and reaction theory[J].Science and Technology Innovation Herald,2009,6(13):4-5,7. | |
| 17 | 付东,王兰芬,吴湘铖.DEA-CO2水溶液的表面张力研究[J].化学学报,2012,70(3):134-139. |
| FU Dong, WANG Lanfen, WU Xiangcheng.Investigation of the surface tension for diethanolamine-CO2 aqueous solutions[J].Acta Chimica Sinica,2012,70(3):134-139. | |
| 18 | 何婷,林文胜.基于余热利用的活化MDEA法脱除CO2的天然气液化系统[J].化工学报,2021,72(S1):453-460. |
| HE Ting, LIN Wensheng.Natural gas liquefaction system with activated MDEA method for CO2 removalbased on waste heat utilization[J].CIESC Journal,2021,72(S1):453-460. | |
| 19 | GAO Hongxia, WU Zeyang, LIU Helei,et al.Experimental studies on the effect of tertiary amine promoters in aqueous monoethanolamine(MEA) solutions on the absorption/stripping performan-ces in post-combustion CO2 capture[J].Energy & Fuels,2017,31(12):13883-13891. |
| 20 | 赵东亚,揭超,李兆敏.醇胺法碳捕集工艺解吸塔能耗分析[J].中国石油大学学报(自然科学版),2016,40(6):150-155. |
| ZHAO Dongya, Chao JIE, LI Zhaomin.Energy consumption analysis of desorption column in amine-based CO2 capture process[J].Journal of China University of Petroleum(Edition of Natural Science),2016,40(6):150-155. | |
| 21 | LV Bihong, GUO Bingsong, ZHOU Zuoming,et al.Mechanisms of CO2 capture into monoethanolamine solution with different CO2 loading during the absorption/desorption processes[J].Environmental Science & Technology,2015,49(17):10728-10735. |
| 22 | SCHÄFFER A, BRECHTEL K, SCHEFFKNECHT G.Comparative study on differently concentrated aqueous solutions of MEA and TETA for CO2 capture from flue gases[J].Fuel,2012,101:148-153. |
| 23 | SAHRAIE S, RASHIDI H, VALEH-E-SHEYDA P.An optimization framework to investigate the CO2 Capture Performance by MEA:Experimental and Statistical studies using Box-Behnken Design[J].Process Safety and Environmental Protection,2018,122:161-168. |
| 24 | AOUINI I, LEDOUX A, ESTEL L,et al.Pilot plant studies for CO2 capture from waste incinerator flue gas using MEA based solvent[J].Oil & Gas Science and Technology-Revue D’IFP Energies Nouvelles,2014,69(6):1091-1104. |
| 25 | SHAHID M Z, MAULUD A S, BUSTAM M A,et al.Packed column modelling and experimental evaluation for CO2 absorption using MDEA solution at high pressure and high CO2 concentrations[J].Journal of Natural Gas Science and Engineering,2021,88:103829. |
| 26 | GLADIS A, GUNDERSEN M T, NEERUP R,et al.CO2 mass transfer model for carbonic anhydrase-enhanced aqueous MDEA solutions[J].Chemical Engineering Journal,2018,335:197-208. |
| 27 | LI Shuhong, DING Yi, ZHANG Xiaosong.Enhancement on CO2 bubble absorption in MDEA solution by TiO2 nanoparticles[J].Advanced Materials Research,2013,631/632:127-134. |
| 28 | 林冠屹,朱春英,付涛涛,等.微通道内MEA/MDEA混合溶液吸收CO2的传质特性[J].化工学报,2018,69(11):4675-4682. |
| LIN Guanyi, ZHU Chunying, FU Taotao,et al.Mass transfer performance of CO2 absorption into aqueous mixture of monoethanolamine with N-methyldiethanolamine in microchannel[J].CIESC Journal,2018,69(11):4675-4682. | |
| 29 | FU Dong, CHEN Lihong, QIN Liguang.Experiment and model for the viscosity of carbonated MDEA-MEA aqueous solutions[J].Fluid Phase Equilibria,2012,319:42-47. |
| 30 | SEMA T, NAAMI A, FU Kaiyun,et al.Comprehensive mass transfer and reaction kinetics studies of CO2 absorption into aqueous solutions of blended MDEA-MEA[J].Chemical Engineering Journal,2012,209:501-512. |
| 31 | LAW L C, YUSOFF AZUDIN N, SHUKOR S R ABD.Optimization and economic analysis of amine-based acid gas capture unit using monoethanolamine/methyl diethanolamine[J].Clean Technologies and Environmental Policy,2018,20(3):451-461. |
| 32 | 陈杰,郭清,花亦怀,等.MDEA+MEA/DEA混合胺液脱碳性能实验研究[J].天然气工业,2014,34(5):137-143. |
| CHEN Jie, GUO Qing, HUA Yihuai,et al.An experimental study of absorption and desorption of blended amine solutions MDEA+MEA/DEA for natural gas decarburization[J].Natural Gas Industry,2014,34(5):137-143. | |
| 33 | 乔琨,吕泽宁,杨立军,等.氨法捕碳技术再生过程无机添加剂效应研究进展[J].无机盐工业,2022,54(10):79-86. |
| QIAO Kun, Zening LÜ, YANG Lijun,et al.Research progress on effect of inorganic additives on regeneration process of carbon capture by ammonia[J].Inorganic Chemicals Industry,2022,54(10):79-86. | |
| 34 | 马双忱,王梦璇,孟亚男,等.氨法吸收烟气中二氧化碳与脱碳后溶液解吸研究进展[J].化工进展,2012,31(5):1143-1148,1159. |
| MA Shuangchen, WANG Mengxuan, MENG Yanan,et al.Research on the absorption of CO2 from flue gas and the desorption of decarbonization solution using ammonia method[J].Chemical Industry and Engineering Progress,2012,31(5):1143-1148,1159. | |
| 35 | CHU Fengming, LIU Yifang, YANG Lijun,et al.Ammonia escape mass transfer and heat transfer characteristics of CO2 absorption in packed absorbing column[J].Applied Energy,2017,205:1596-1604. |
| 36 | 高建民,郑张,杨建国,等.一种新型氨法脱碳工艺的流程模拟与能耗分析[J].中国电机工程学报,2016,36(5):1315-1321. |
| GAO Jianmin, ZHENG Zhang, YANG Jianguo,et al.Process simulation and analysis of energy consumption for a new technology of carbon capture[J].Proceedings of the CSEE,2016,36(5):1315-1321. | |
| 37 | JILVERO H, NORMANN F, ANDERSSON K,et al.Heat requirement for regeneration of aqueous ammonia in post-combustion carbon dioxide capture[J].International Journal of Greenhouse Gas Control,2012,11:181-187. |
| 38 | ZHANG Minkai, GUO Yincheng.Rate based modeling of absorption and regeneration for CO2 capture by aqueous ammonia solution[J].Applied Energy,2013,111:142-152. |
| 39 | NIU Zhenqi, GUO Yincheng, ZENG Qing,et al.Experimental studies and rate-based process simulations of CO2 absorption with aqueous ammonia solutions[J].Industrial & Engineering Chemistry Research,2012,51(14):5309-5319. |
| 40 | HUANG Houping, CHANG S G, DORCHAK T.Method to regenerate ammonia for the capture of carbon dioxide[J].Energy & Fuels,2002,16(4):904-910. |
| 41 | 袁标,沈鹏.离子液体捕集CO2的研究进展[J].天然气化工—C1化学与化工,2022,47(3):1-7. |
| YUAN Biao, SHEN Peng.Research progress of CO2 capture by ionic liquids[J].Natural Gas Chemical Industry,2022,47(3):1-7. | |
| 42 | BATES E D, MAYTON R D, NTAI I,et al.CO2 capture by a task-specific ionic liquid[J].Journal of the American Chemical Society,2002,124(6):926-927. |
| 43 | 张慧,张红梅,沈锦优,等.氨基功能型离子液体吸收CO2的性能[J].化工学报,2016,67(12):5057-5065. |
| ZHANG Hui, ZHANG Hongmei, SHEN Jinyou,et al.Absorption performance of CO2 in amino-functionalized task-specific ionic liquids[J].CIESC Journal,2016,67(12):5057-5065. | |
| 44 | LV Bihong, JING Guohua, QIAN Yuhao,et al.An efficient absorbent of amine-based amino acid-functionalized ionic liquids for CO2 capture:High capacity and regeneration ability[J].Chemical Engineering Journal,2016,289:212-218. |
| 45 | XUE Zhimin, ZHANG Zhaofu, HAN Jin,et al.Carbon dioxide capture by a dual amino ionic liquid with amino-functionalized imidazolium cation and taurine anion[J].International Journal of Greenhouse Gas Control,2011,5(4):628-633. |
| 46 | BHATTACHARYYA S, SHAH F U.Ether functionalized choline tethered amino acid ionic liquids for enhanced CO2 capture[J].ACS Sustainable Chemistry & Engineering,2016,4(10):5441-5449. |
| 47 | WANG Congmin, LUO Huimin, JIANG Deen,et al.Carbon dioxide capture by superbase-derived protic ionic liquids[J].Angewandte Chemie,2010,49(34):5978-5981. |
| 48 | LI Fangfang, BAI Yinge, ZENG Shaojuan,et al.Protic ionic liquids with low viscosity for efficient and reversible capture of carbon dioxide[J].International Journal of Greenhouse Gas Control,2019,90:102801. |
| 49 | 涂智芳,魏建文,周小斌.固-液相变二氧化碳吸收剂的研究进展[J].洁净煤技术,2022,28(9):122-132. |
| TU Zhifang, WEI Jianwen, ZHOU Xiaobin.Research progress on carbon dioxide capture using solid-liquid phase-change absorbents[J].Clean Coal Technology,2022,28(9):122-132. | |
| 50 | 王展旭,王敏.液-液相变溶剂混合过程的数值模拟[J].当代化工,2019,48(4):825-829. |
| WANG Zhanxu, WANG Min.Numerical simulation on the mixing process of liquid-liquid phase change solvents[J].Contemporary Chemical Industry,2019,48(4):825-829. | |
| 51 | ZHANG Jiafei, QIAO Yu, WANG Wanzhong,et al.Development of an energy-efficient CO2 capture process using thermomorphic biphasic solvents[J].Energy Procedia,2013,37:1254-1261. |
| 52 | RAYNAL L, ALIX P, BOUILLON P A,et al.The DMX™ process:An original solution for lowering the cost of post-combustion carbon capture[J].Energy Procedia,2011,4:779-786. |
| 53 | ZHOU Haicheng, XU Xin, CHEN Xiaochun,et al.Novel ionic liquids phase change solvents for CO2 capture[J].International Journal of Greenhouse Gas Control,2020,98:103068 |
| 54 | ZHANG Weidong, JIN Xianhang, TU Weiwei,et al.Development of MEA-based CO2 phase change absorbent[J].Applied Energy,2017,195:316-323. |
| 55 | 张卫风,周武,王秋华.相变吸收捕集烟气中CO2技术的发展现状[J].化工进展,2022,41(4):2090-2101. |
| ZHANG Weifeng, ZHOU Wu, WANG Qiuhua.Recent developments of phase-change absorption technology for CO2 capture from flue gas[J].Chemical Industry and Engineering Progress,2022,41(4):2090-2101. | |
| 56 | WANG Lidong, AN Shanlong, YU Songhua,et al.Mass transfer characteristics of CO2 absorption into a phase-change solvent in a wetted-wall column[J].International Journal of Greenhouse Gas Control,2017,64:276-283. |
| 57 | FERNANDEZ S E, HEFFEMAN K, HAM D V V L.Conceptual design of a novel CO2 capture process based on precipitating amino acid solvents[J].Industrial Engineering Chemistry Research,2013,52(34):12223-12235. |
| 58 | ZHOU Qiang, LIU Lan, CROISET E,et al.Modeling study of the heat of absorption and solid precipitation for CO2 capture by chilled ammonia[J].RSC Advances,2019,9(35):20075-20086. |
| 59 | BAK C U, ASIF M, KIM W S.Experimental study on CO2 capture by chilled ammonia process[J].Chemical Engineering Journal,2015,265:1-8. |
| 60 | AUGUSTSSON O, BABURAO B, DUBE S,et al.Chilled ammonia process scale-up and lessons learned[J].Energy Procedia,2017,114:5593-5615. |
| 61 | TELIKAPALLI V, KOZAK F, FRANCOIS J,et al.CCS with the Alstom chilled ammonia process development program-field pilot results[J].Energy Procedia,2011,4:273-281. |
| 62 | LI Jingde, CHENG Kuang, CROISET E,et al.Effects of SO2 on CO2 capture using chilled ammonia solvent[J].International Journal of Greenhouse Gas Control,2017,63:442-448. |
| 63 | DARDE V, THOMSEN K, VAN WELL W J M,et al.Chilled ammonia process for CO2 capture[J].International Journal of Greenhouse Gas Control,2010,4(2):131-136. |
| 64 | ANDERSON C, HARKIN T,HO M,et al.Developments in the CO2 CRC UNO MK3 process:A multi-component solvent process for large scale CO2 capture[J].Energy Procedia,2013,37:225- 232. |
| 65 | TAO Mengna, GAO Jinzhe, ZHANG Pei,et al.Biogas upgrading by capturing CO2 in non-aqueous phase-changing diamine solutions[J].Energy & Fuels,2017,31(6):6298-6304. |
| [1] | MA Shuqing, LI Changwen, SHI Chenglong, QIN Yaru. Kinetic study of lithium extraction from solution with iron-based ionic liquid system [J]. Inorganic Chemicals Industry, 2024, 56(9): 60-66. |
| [2] | ZHOU Wanji, LI Sixia. Study on extraction lithium from brine with high magnesium using ionic liquid/metal salt extraction system [J]. Inorganic Chemicals Industry, 2024, 56(9): 54-59. |
| [3] | LU Nana, QIN Yaru, MA Shuqing, WANG Qihui, LIU Bing, SHI Chenglong. Study on extraction of lithium from simulated old brine of salt lake by pyridine ionic liquid system [J]. Inorganic Chemicals Industry, 2023, 55(7): 45-50. |
| [4] | LAI Xiaoling, ZHOU Wei, ZANG Jiazhong, DONG Zichao, HU Mingliang, SUN Shaochen. Research progress of low temperature cyclic sorption of carbon dioxide by calcium oxide-based sorbents [J]. Inorganic Chemicals Industry, 2023, 55(5): 16-23. |
| [5] | JI Hongfeng, LI Canhua, DU Gang, ZHANG Yongzhu, XU Wenzhen, LI Minghui. Research progress of preparation and sintering resistance of calcium-based CO2 trapping materials from solid waste sources [J]. Inorganic Chemicals Industry, 2023, 55(3): 28-35. |
| [6] | HUA Wen,LÜ Ruiliang. Application status and development direction of wet flue gas desulfurization technology [J]. Inorganic Chemicals Industry, 2022, 54(12): 10-18. |
| [7] | KANG Jin,WEI Lina,CHENG Huaigang. Research progress on application of ionic liquids in extracting lithium from salt lakes [J]. Inorganic Chemicals Industry, 2022, 54(1): 1-6. |
| [8] | Wang Jia,Liu Jinyan,Gao Jun,Wang Lu,Wang Yongli. Study on properties and extraction efficiency of quaternary phosphonium salt ionic liquid/Surfactant/salt aqueous two phase systems [J]. Inorganic Chemicals Industry, 2021, 53(4): 43-47. |
| [9] | Li Yiyi,Sun Qiaoyi,Ma Linge,Zhuo Jinde,Song Weiguo. Study on mineralization-desorption and regeneration of post-CO2 capture by glycine salt [J]. Inorganic Chemicals Industry, 2021, 53(2): 38-41. |
| [10] | Qin Yaru,Shi Chenglong,Wang Xingquan,Song Guixiu,Li Hongxia,Zhang Jingjing. Study on scrubbing process of lithium extraction from brine in ionic liquid system [J]. Inorganic Chemicals Industry, 2020, 52(3): 55-58. |
| [11] | Liu Bingguang,Zu Xiaodong,Li Jiansheng,Lu Junfeng,Wang Zejiang. Research progress in supported lithium ion sieve absorbents [J]. Inorganic Chemicals Industry, 2019, 51(9): 12-16. |
| [12] | Shi Chunhui1,Lü Pin1,Huang Xia2,Zhong Jianchu2. Study on hydrothermal synthesis of CaB6O10·5H2O and its phase transition at high tempareture [J]. Inorganic Chemicals Industry, 2019, 51(7): 48-50. |
| [13] | LIAO Xue-Feng, CHEN Guo, LIU Qian-Qian, CHANG Xiao-Dong, ZHANG Li-Bo, PENG Jin-Hui. Recent advances in phase transition of TiO2 [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(8): 6-. |
| [14] | ZHAO Yun, YANG Xu, DAN Jian-Ming, QIAO Xiu-Wen, LI Hong-Ling, HONG Cheng-Lin. Study on preparation of silica nanoparticles by silicon tetrachloride with air blowing method [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(8): 28-. |
| [15] | Sun Ming;Yu Lin;Peng Lanqiao;Wang Xuetao;Yu Qian;Yu Jian;Hao Zhifeng. Synthesis of nickel boride in ionic liquid and research on catalytic hydrogenation performance thereof [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(9): 0-0. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||