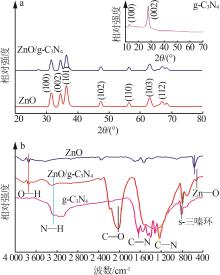
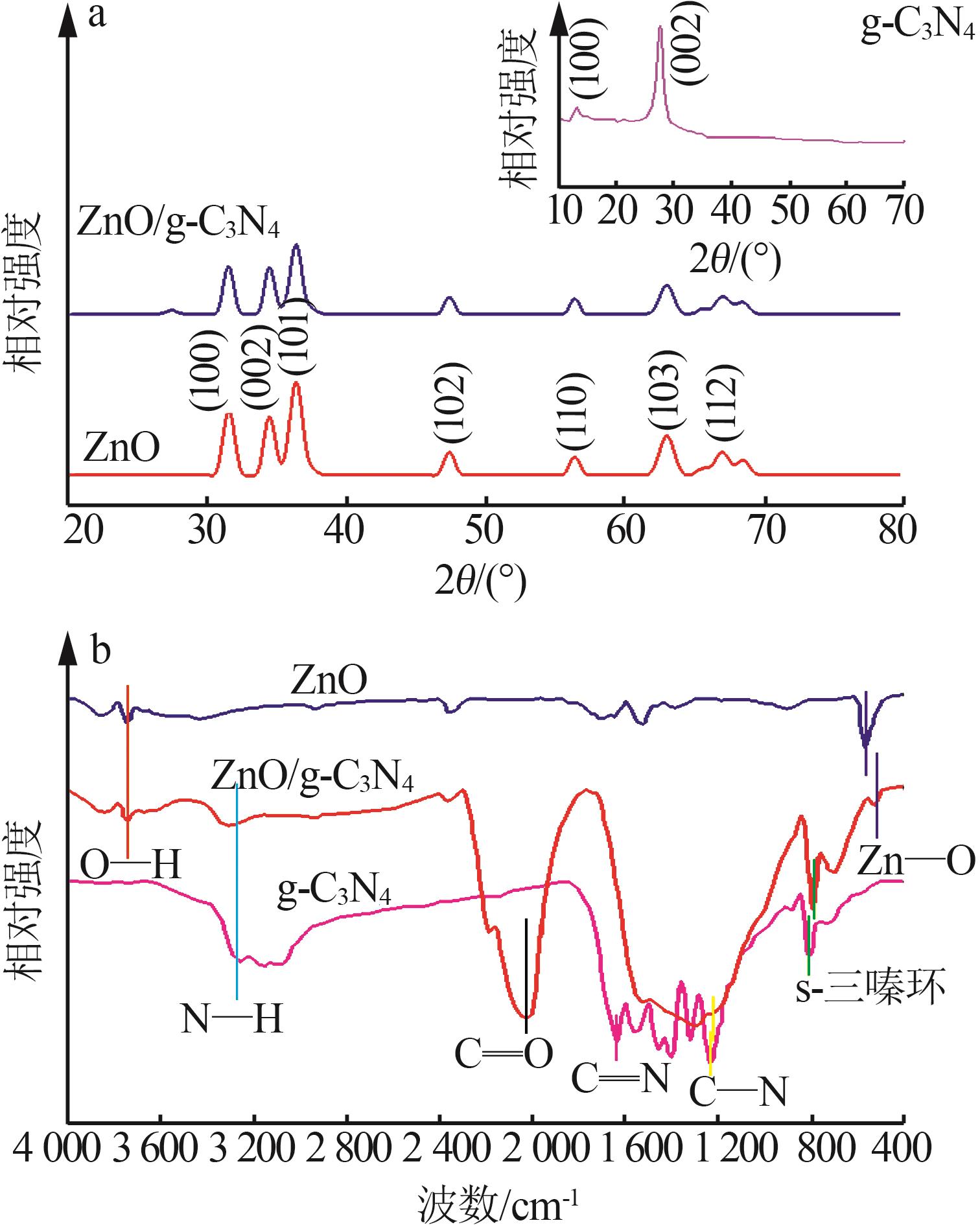
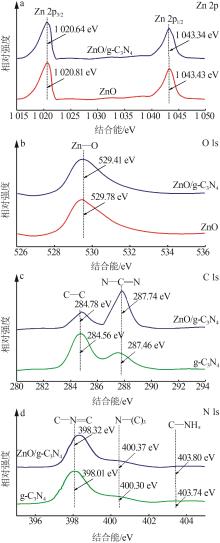
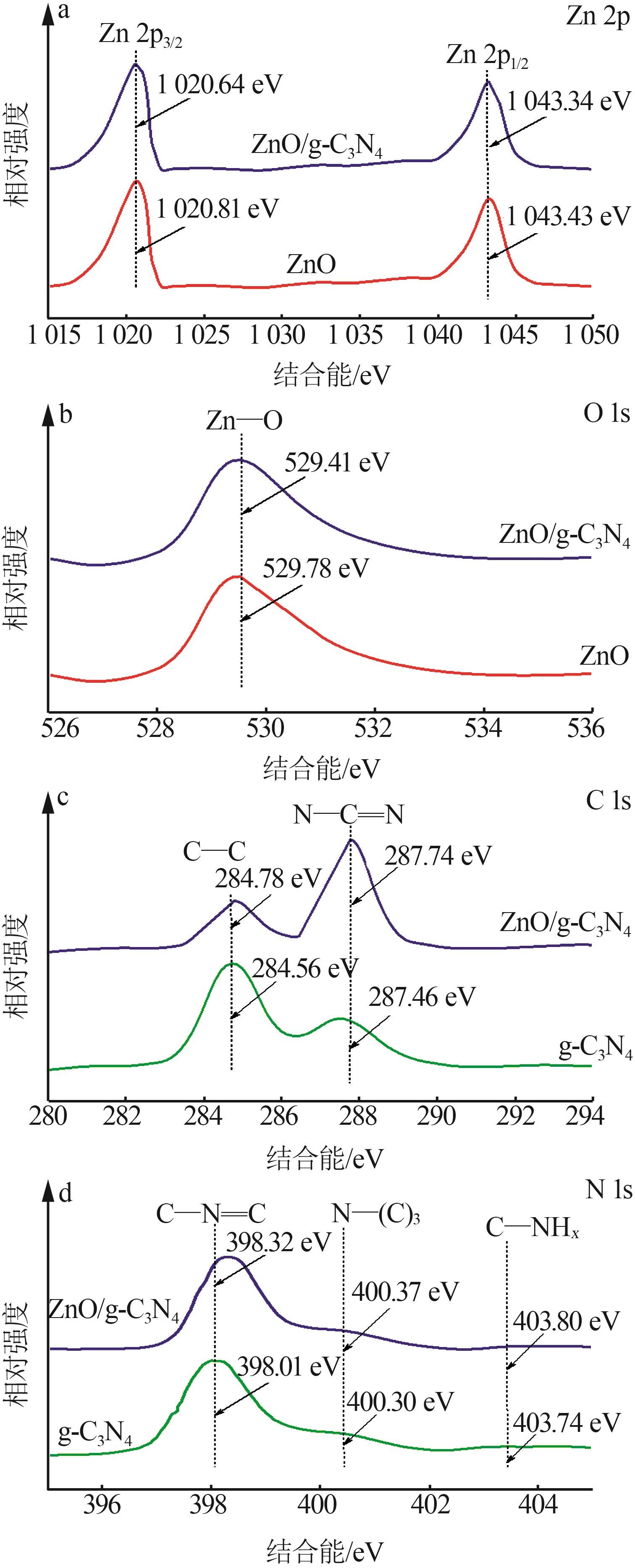
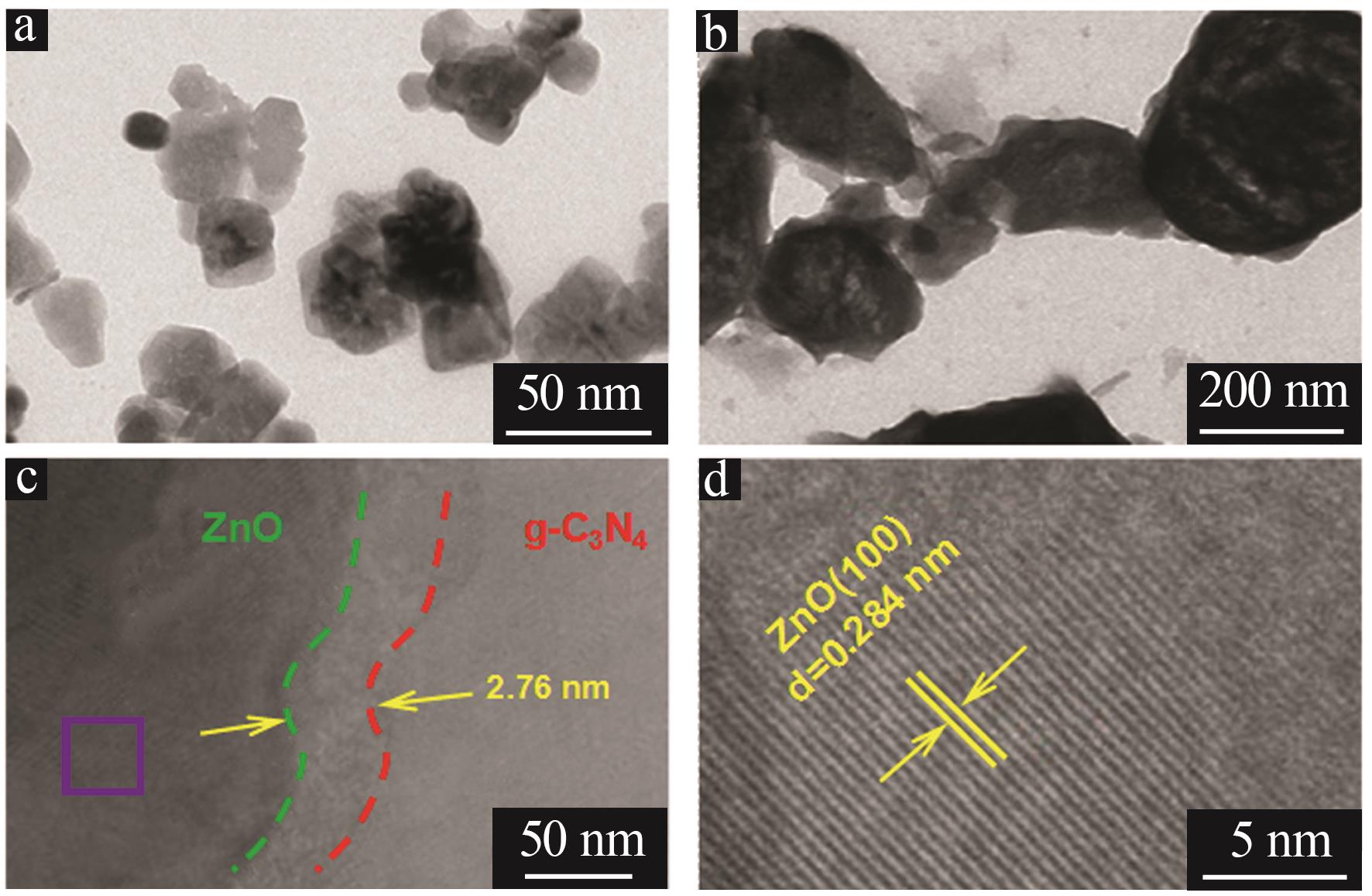
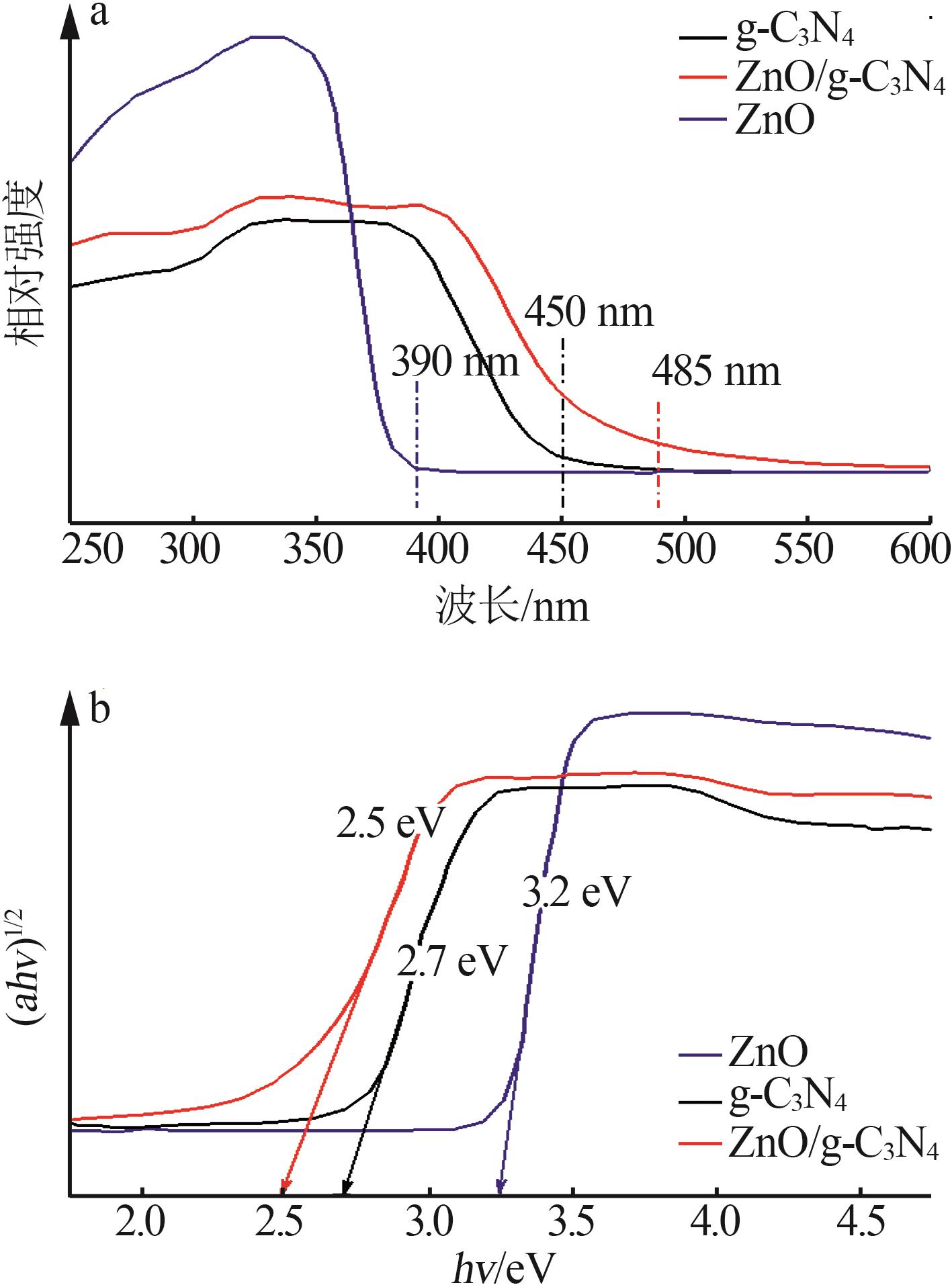
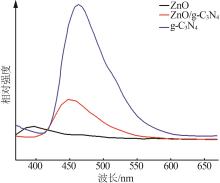
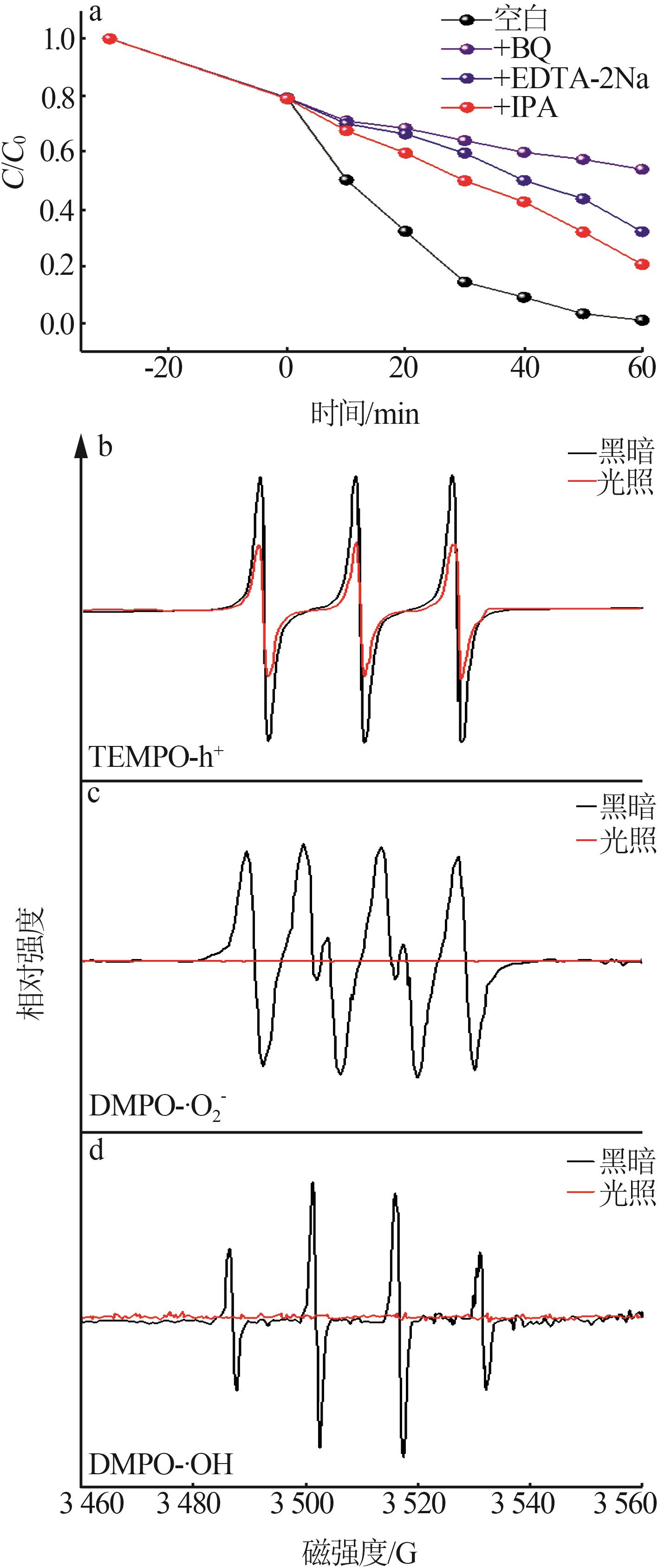
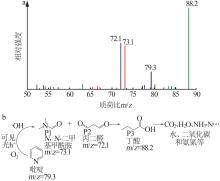
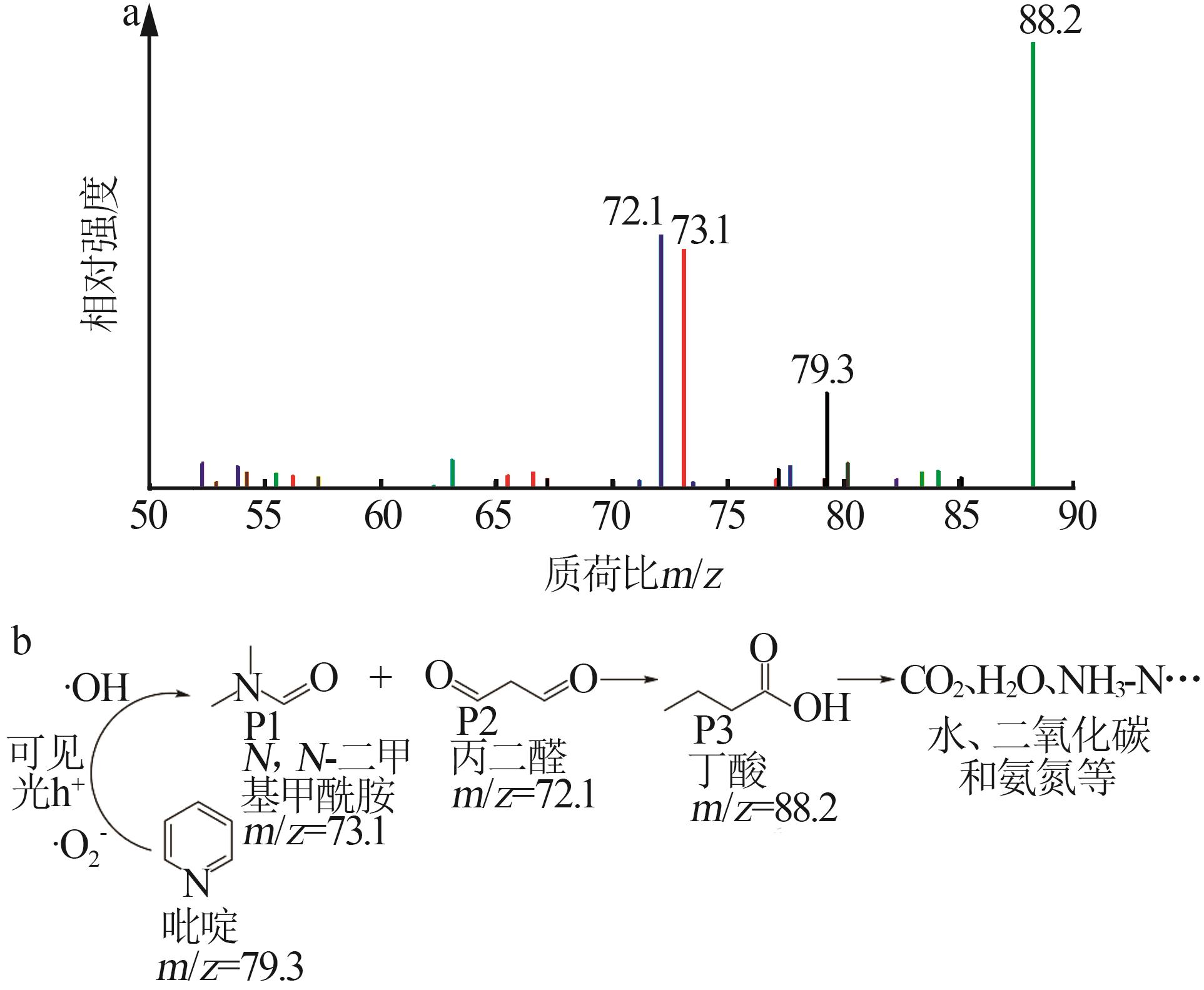
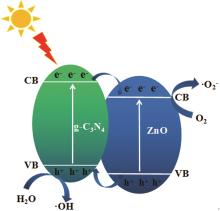
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (4): 133-142.doi: 10.19964/j.issn.1006-4990.2023-0527
• Catalytic Materials • Previous Articles Next Articles
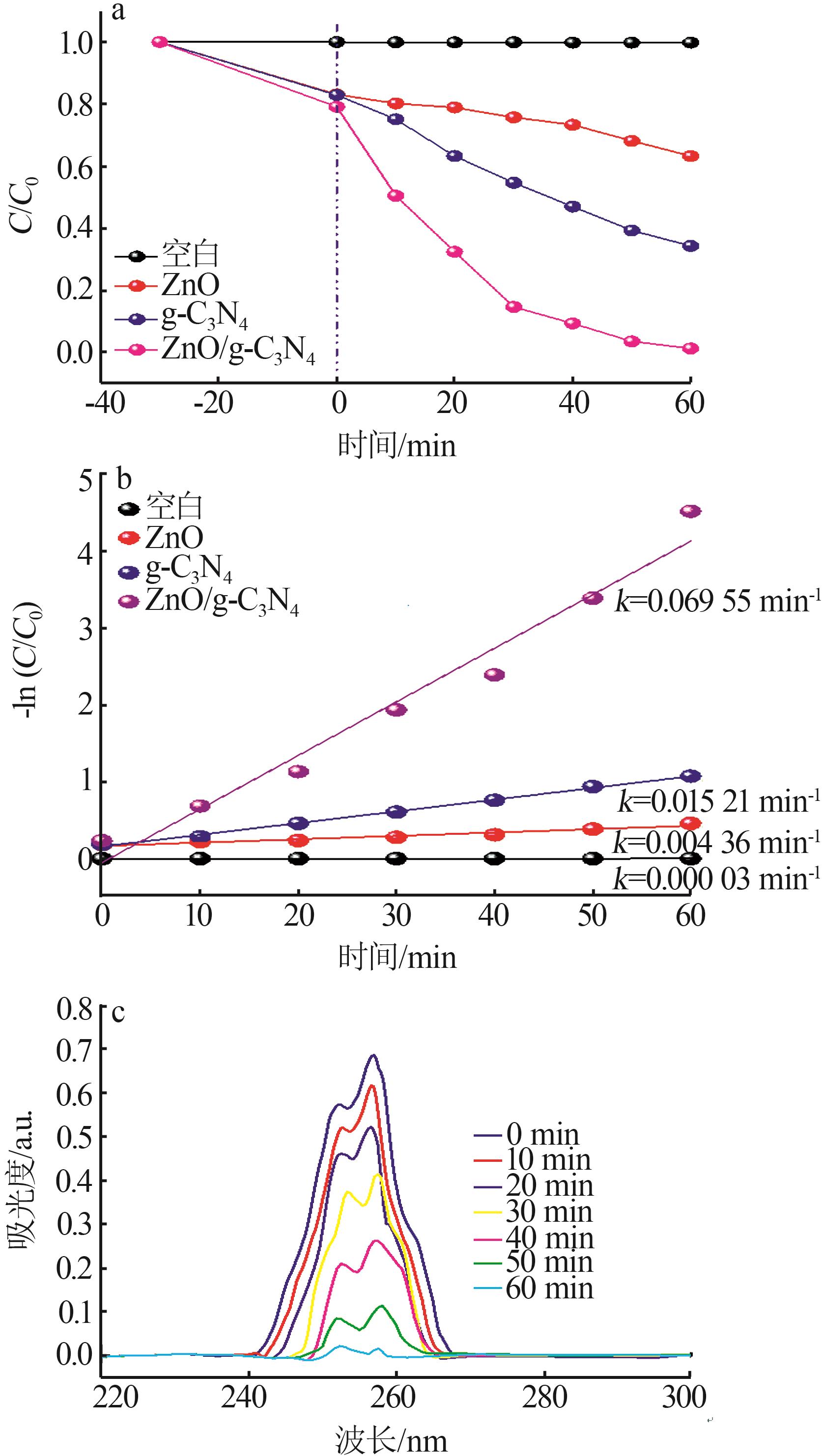
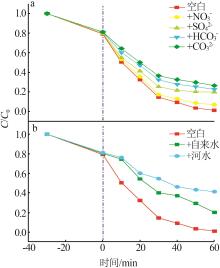
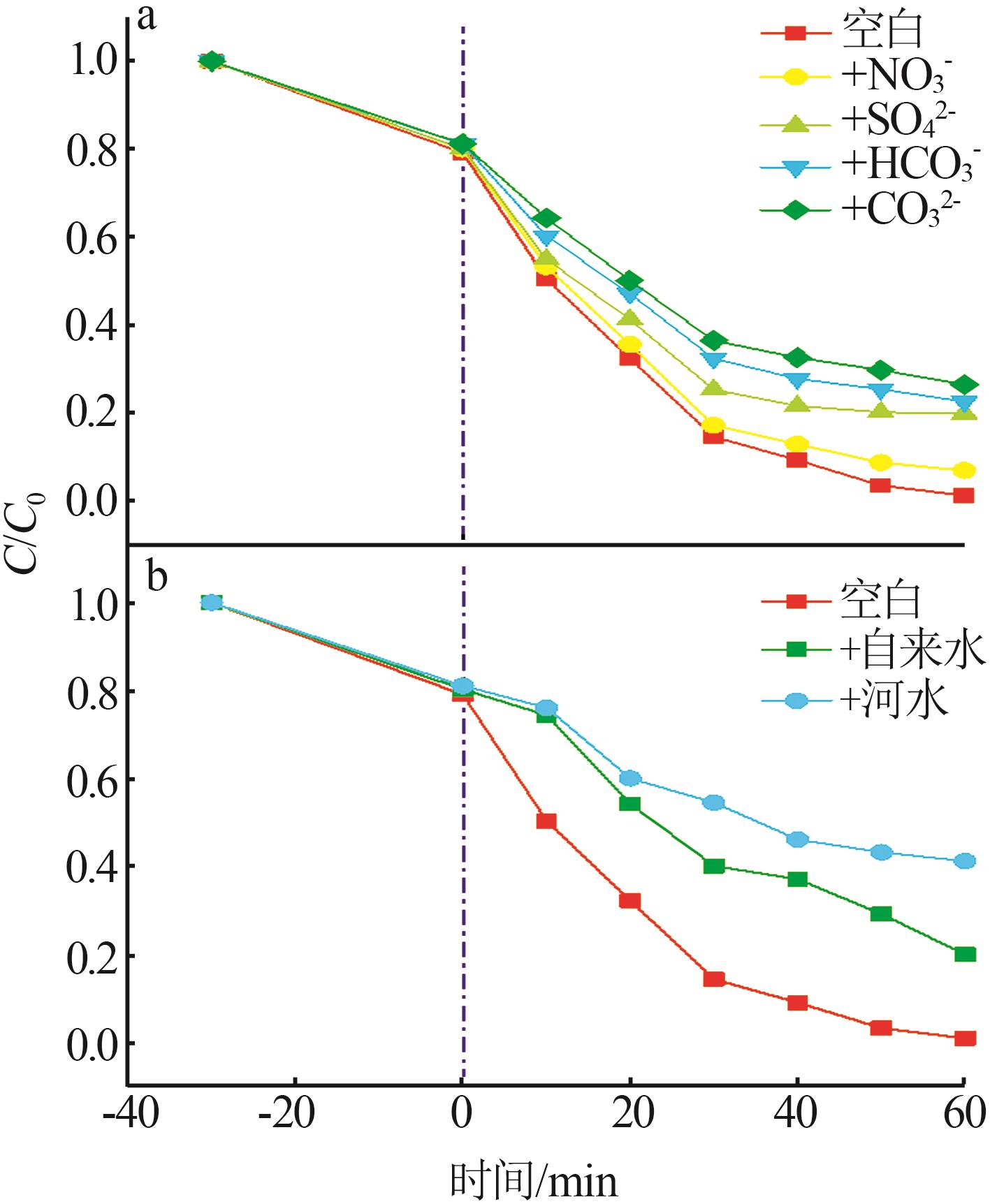
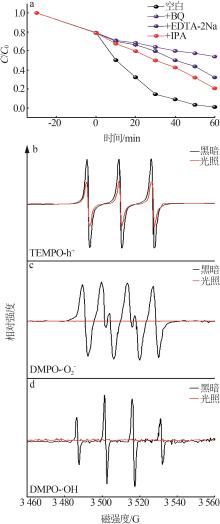
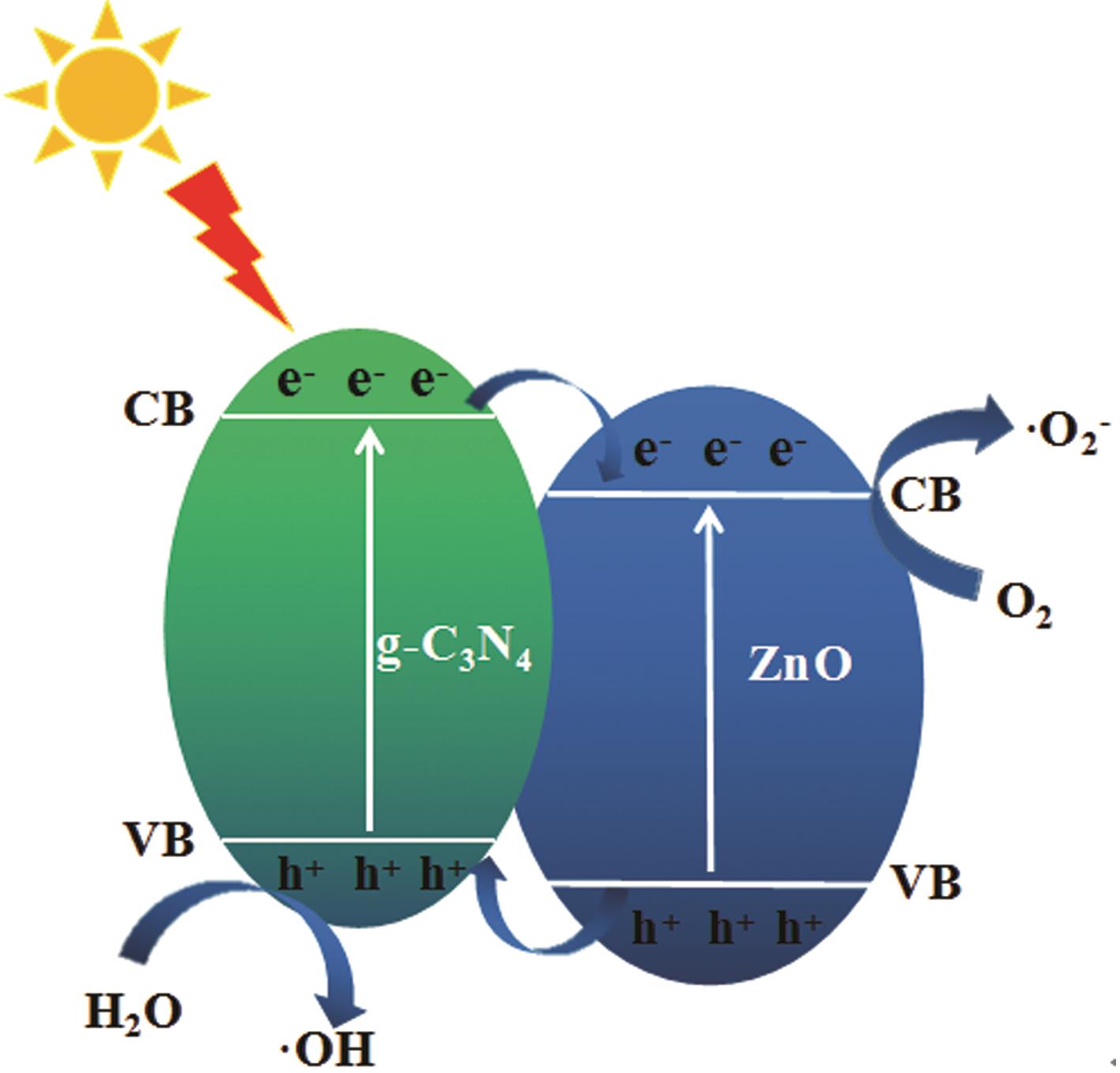
Preparation of ZnO/g-C3N4 heterojunction photocatalytic material and its degradation of pyridine
- Henan Vocational College of Applied Technology,Zhengzhou 450042,China
-
Received:2023-11-06Online:2024-04-10Published:2024-04-18
CLC Number:
Cite this article
TANG Bei. Preparation of ZnO/g-C3N4 heterojunction photocatalytic material and its degradation of pyridine[J]. Inorganic Chemicals Industry, 2024, 56(4): 133-142.
share this article
Table 1
Orthogonal experimental results"
| 次数 | 因素 | 吡啶降 解率/% | |||
|---|---|---|---|---|---|
| 催化剂投加量/mg | m(ZnO)∶ m(g-C3N4) | 吡啶初始质量 浓度/(mg·L-1) | 体系 pH | ||
| 1 | 40 | 1∶2 | 10 | 6.0 | 92.3 |
| 2 | 40 | 1∶1 | 20 | 7.0 | 92.1 |
| 3 | 40 | 1∶3 | 30 | 8.0 | 89.8 |
| 4 | 50 | 1∶2 | 20 | 8.0 | 96.2 |
| 5 | 50 | 1∶1 | 30 | 6.0 | 89.6 |
| 6 | 50 | 1∶3 | 10 | 7.0 | 90.5 |
| 7 | 60 | 1∶2 | 30 | 7.0 | 91.3 |
| 8 | 60 | 1∶1 | 10 | 8.0 | 86.7 |
| 9 | 60 | 1∶3 | 20 | 6.0 | 89.5 |
| 验证 | 50 | 1∶2 | 20 | 7.0 | 98.9 |
| 均值1 | 91.400 | 93.267 | 89.833 | 90.467 | |
| 均值2 | 92.100 | 89.467 | 92.600 | 91.300 | |
| 均值3 | 89.167 | 89.933 | 90.233 | 90.900 | |
| 极差 | 2.933 | 3.800 | 2.767 | 0.833 | |
Table 1
| 1 | 李启辉. 煤化工废水处理及资源化利用研究现状[J]. 应用化工, 2023, 52(7):2234-2238,2243. |
| LI Qihui. Research progress on treatment and comprehensive utilization of coal chemical wastewater[J]. Applied Chemical Industry, 2023, 52(7):2234-2238,2243. | |
| 2 | 马鸣, 张新妙, 章晨林. 现代煤化工高盐废水处理技术研究进展[J]. 石油化工, 2023, 52(3):427-432. |
| MA Ming, ZHANG Xinmiao, ZHANG Chenlin. Research progress on treatment technologies of high-salinity wastewater from modern coal chemical industry[J]. Petrochemical Technology, 2023, 52(3):427-432. | |
| 3 | 谢娟, 夏润南, 赵树春, 等. ZnO/煤矸石复合光催化剂的制备与性能研究[J]. 矿产综合利用, 2019(4):126-129,116. |
| XIE Juan, XIA Runnan, ZHAO Shuchun, et al. Preparation and properties of ZnO/coal gangue composite photocatalysts[J]. Multipurpose Utilization of Mineral Resources, 2019(4):126-129,116. | |
| 4 |
LIAO Quan, RONG Hongwei, ZHAO Meihua, et al. Interaction between tetracycline and microorganisms during wastewater treatment:A review[J]. Science of the Total Environment, 2021, 757:143981.
doi: 10.1016/j.scitotenv.2020.143981 |
| 5 |
陈莉荣, 宛艳玉, 王瑞, 等. UV-TiO2-PS光催化降解煤化工废水中含氮有机物[J]. 化工环保, 2023, 43(3):313-318.
doi: 10.3969/j.issn.1006-1878.2023.03.005 |
|
CHEN Lirong, WAN Yanyu, WANG Rui, et al. Photocatalytic degradation of nitrogen-containing organic compounds in coal chemical wastewater by UV-TiO2-PS system[J]. Environmental Protection of Chemical Industry, 2023, 43(3):313-318.
doi: 10.3969/j.issn.1006-1878.2023.03.005 |
|
| 6 | 冯琪淋. 可见光催化-微生物直接耦合技术降解吡啶的机理研究[D]. 南宁: 广西大学, 2022. |
| FENG Qilin. Study on the mechanism of pyridine degradation by visible light photocatalysis-microorganism direct coupling technology[D]. Nanning: Guangxi university, 2022. | |
| 7 |
JIANG Lei, WEN Yanyi, ZHU Zhongjie, et al. A double cross-linked strategy to construct graphene aerogels with highly efficient methylene blue adsorption performance[J]. Chemosphere, 2021, 265:129169.
doi: 10.1016/j.chemosphere.2020.129169 |
| 8 |
PARK B H, KIM S, SEO A Y, et al. Evaluation of optimal conditions for anionic surfactant removal in wastewater[J]. Chemosphere, 2021, 263:128174.
doi: 10.1016/j.chemosphere.2020.128174 |
| 9 | 麻微微, 施雪卿, 孔巧平, 等. 微电解耦合同步硝化反硝化工艺强化煤化工废水脱氮效能及微生物群落结构研究[J]. 环境科学学报, 2022, 42(3):130-140. |
| MA Weiwei, SHI Xueqing, KONG Qiaoping, et al. Nitrogen removal efficiency and microbial community characteristics in microelectrolysis coupled simultaneous nitrification and denitrification process for coal chemical wastewater treatment[J]. Acta Scientiae Circumstantiae, 2022, 42(3):130-140. | |
| 10 | 张文, 林长喜, 彭永臻. 现代煤化工废水近零排放技术集成与优化建议[J]. 环境工程, 2021, 39(11):41-45,109. |
| ZHANG Wen, LIN Changxi, PENG Yongzhen. Recommendations on integration and optimization of near-zero discharge technology for wastewater from modern coal chemical industry[J]. Environmental Engineering, 2021, 39(11):41-45,109. | |
| 11 | 解彬, 宋泽宇, 雷珂, 等. 关于实现煤化工废水零排放工艺设计的研究[J]. 应用化工, 2021, 50(9):2534-2539. |
| XIE Bin, SONG Zeyu, LEI Ke, et al. Research on process design of zero discharge of coal chemical wastewater[J]. Applied Chemical Industry, 2021, 50(9):2534-2539. | |
| 12 | 孙海杰, 刘欣改, 陈志浩, 等. BiOI/g-C3N4光催化降解甲基橙研究[J]. 无机盐工业, 2021, 53(4):90-94. |
| SUN Haijie, LIU Xin′gai, CHEN Zhihao, et al. Study on photocatalytic degradation of methyl orange by BiOI/g-C3N4 [J]. Inorganic Chemicals Industry, 2021, 53(4):90-94. | |
| 13 |
QU Zhengjun, JING Zhenyang, CHEN Xiaoming, et al. Preparation and photocatalytic performance study of dual Z-scheme Bi2Zr2O7/g-C3N4/Ag3PO4 for removal of antibiotics by visible-light[J]. Journal of Environmental Sciences, 2023, 125:349-361.
doi: 10.1016/j.jes.2022.01.010 pmid: 36375920 |
| 14 |
LIU Yuanyuan, TIAN Jing, WEI Linyu, et al. Modified g-C3N4/TiO2/CdS ternary heterojunction nanocomposite as highly visible light active photocatalyst originated from CdS as the electron source of TiO2 to accelerate Z-type heterojunction[J]. Separation and Purification Technology, 2021, 257:117976.
doi: 10.1016/j.seppur.2020.117976 |
| 15 |
WANG Xunhe, HE Meilin, Zhaodong NAN. Effects of adsorption capacity and activity site on Fenton-like catalytic performance for Na and Fe co-doped g-C3N4 [J]. Separation and Purification Technology, 2021, 256:117765.
doi: 10.1016/j.seppur.2020.117765 |
| 16 | 董伟, 常启明, 王文博, 等. g-C3N4纳米片的剥离制备方法研究进展[J]. 硅酸盐学报, 2023, 51(7):1868-1882. |
| DONG Wei, CHANG Qiming, WANG Wenbo, et al. Research progress in preparing methods of g-C3N4 nanosheets by exfoliation[J]. Journal of the Chinese Ceramic Society, 2023, 51(7):1868-1882. | |
| 17 | 陈彰旭, 朱丹琛, 傅明连. g-C3N4/TiO2复合材料制备及其处理罗丹明B研究[J]. 无机盐工业, 2023, 55(7):130-136. |
| CHEN Zhangxu, ZHU Danchen, FU Minglian. Study on preparation of g-C3N4/TiO2 composites and application for rhodamine B removal[J]. Inorganic Chemicals Industry, 2023, 55(7):130- 136. | |
| 18 | 陈建军, 周诗园, 黄雨晨, 等. 无定型碳/g-C3N4制备及其光催化降解四环素性能[J]. 化学试剂, 2023, 45(7):107-112. |
| CHEN Jianjun, ZHOU Shiyuan, HUANG Yuchen, et al. Preparation of amorphous carbon/g-C3N4 and its photocatalytic degradation of tetracycline[J]. Chemical Reagents, 2023, 45(7):107- 112. | |
| 19 | 汲畅, 王国胜. Ag3PO4/g-C3N4异质结催化剂可见光降解黄连素[J]. 无机盐工业, 2022, 54(4):175-180. |
| JI Chang, WANG Guosheng. Degradation of berberine by visible light over Ag3PO4/g-C3N4 heterojunction catalyst[J]. Inorganic Chemicals Industry, 2022, 54(4):175-180. | |
| 20 | 卜义夫, 刘思乐, 杜文娟, 等. 二硫化钼/石墨相氮化碳光催 |
| 化降解印染废水研究[J]. 皮革科学与工程, 2023, 33(3):12- 18. | |
| BU Yifu, LIU Sile, DU Wenjuan, et al. Study on photocatalytic degradation of printing and dyeing wastewater by molybdenum disulfide/graphite carbon nitride[J]. Leather Science and Engineering, 2023, 33(3):12-18. | |
| 21 | 黄曦瑶, 李明春, 郭银彤. g-C3N4/Bi/Bi2WO6光催化材料的协同改性研究[J]. 无机盐工业, 2022, 54(12):133-138. |
| HUANG Xiyao, LI Mingchun, GUO Yintong. Study on synergistic modification of g-C3N4/Bi/Bi2WO6 photocatalyst[J]. Inorganic Chemicals Industry, 2022, 54(12):133-138. | |
| 22 | 曾楠, 柴希娟, 胡玲华, 等. ZnO复合石墨相氮化碳纳米片的制备及其光催化性能研究[J]. 材料科学与工艺, 2023, 31(4):41-50. |
| ZENG Nan, CHAI Xijuan, HU Linghua, et al. Preparation and photocatalytic properties of ZnO composite graphitic carbon nitride nanosheets[J]. Materials Science and Technology, 2023, 31(4):41-50. | |
| 23 |
谢锋, 郭建峰, 王海涛, 等. ZnO/CdS/Ag复合光催化剂的制备及其催化和抗菌性能[J]. 材料研究学报, 2023, 37(1):10-20.
doi: 10.11901/1005.3093.2021.586 |
|
XIE Feng, GUO Jianfeng, WANG Haitao, et al. Construction of ZnO/CdS/Ag composite photocatalyst and its catalytic and antibacterial performance[J]. Chinese Journal of Materials Research, 2023, 37(1):10-20.
doi: 10.11901/1005.3093.2021.586 |
|
| 24 |
XU Peng, WANG Peng, WANG Qiao, et al. Facile synthesis of Ag2O/ZnO/rGO heterojunction with enhanced photocatalytic activity under simulated solar light:Kinetics and mechanism[J]. Journal of Hazardous Materials, 2021, 403:124011.
doi: 10.1016/j.jhazmat.2020.124011 |
| 25 |
SILVA F F DA, SILVA R B DA, SILVA T R, et al. Boosting the photocatalytic activity of g-C3N4/ZnO heterojunctions through optimal control of mass ratio[J]. Solid State Sciences, 2023, 138:107128.
doi: 10.1016/j.solidstatesciences.2023.107128 |
| 26 |
TAN Xu, WANG Xing, HANG He, et al. Self-assembly method assisted synthesis of g-C3N4/ZnO heterostructure nanocomposites with enhanced photocatalytic performance[J]. Optical Materials, 2019, 96:109266.
doi: 10.1016/j.optmat.2019.109266 |
| 27 |
VINOTH S, SUBRAMANI K, ONG W J, et al. CoS2 engulfed ultra-thin S-doped g-C3N4 and its enhanced electrochemical performance in hybrid asymmetric supercapacitor[J]. Journal of Colloid and Interface Science, 2021, 584:204-215.
doi: 10.1016/j.jcis.2020.09.071 |
| 28 | LIU Dong, YAO Jia, CHEN Shentao, et al. Construction of rGO-coupled C3N4/C3N5 2D/2D Z-scheme heterojunction to accelerate charge separation for efficient visible light H2 evolution[J]. Applied Catalysis B:Environmental, 2022, 318:121822. |
| 29 |
SUN Qi, SUN Ying, ZHOU Miya, et al. A 2D/3D g-C3N4/ZnO heterojunction enhanced visible-light driven photocatalytic activity for sulfonamides degradation[J]. Ceramics International, 2022, 48(5):7283-7290.
doi: 10.1016/j.ceramint.2021.11.289 |
| 30 |
ZHANG Zhengyi, SUN Yangshan, WANG Youle, et al. Synthesis and photocatalytic activity of g-C3N4/ZnO composite microspheres under visible light exposure[J]. Ceramics International, 2022, 48(3):3293-3302.
doi: 10.1016/j.ceramint.2021.10.104 |
| 31 | WANG Libo, CHENG Bei, ZHANG Liuyang, et al. In situ irradiated XPS investigation on S-scheme TiO2@ZnIn2S4 photocatalyst for efficient photocatalytic CO2 reduction[J]. Small, 2021, 17(41): e2103447. |
| 32 |
VAHABIRAD S, NEZAMZADEH-EJHIEH A. Co-precipitation synthesis of BiOI/(BiO)2CO3:Brief characterization and the kinetic study in the photodegradation and mineralization of sulfas-alazine[J]. Journal of Solid State Chemistry, 2022, 310:123018.
doi: 10.1016/j.jssc.2022.123018 |
| [1] | HUANG Jianan, LU Xiaoyu, WANG Mitang. Effect of Ba-La co-doping on degradation of methylene blue dye by TaON [J]. Inorganic Chemicals Industry, 2024, 56(2): 146-151. |
| [2] | YANG Bo, LIANG Zhiyan, LIU Wenyuan, CAO Jiazhen, LIU Xinyue, XING Mingyang. Research progress of application of molybdenum-based catalytic materials for water pollution control [J]. Inorganic Chemicals Industry, 2023, 55(8): 1-12. |
| [3] | YU Hongchao, ZHANG Mengmeng, JIN Tianxiang. Research progress of microstructure and crystal surface effect of Ag3PO4 photocatalysts [J]. Inorganic Chemicals Industry, 2023, 55(8): 13-20. |
| [4] | ZHAO Yan, HAO Xuewei, SHI Hainan, LI Jiahui, LI Keyan, GUO Xinwen. Study on photocatalytic CO2 reduction performance of Cu-doped TiO2/PCN heterojunction [J]. Inorganic Chemicals Industry, 2023, 55(8): 21-27. |
| [5] | SUN Haijie, CHENG Yuan, TIAN Yuan, LIU Hongyan, CHEN Zhihao. Preparation of BiOI/g-C3N4 catalyst and its photocatalytic degradation performance of Rhodamine B [J]. Inorganic Chemicals Industry, 2023, 55(8): 36-44. |
| [6] | SONG Zhijia, WANG Suisui, KUANG Qin. Hollow Cu-doped TiO2 for enhancing photocatalytic CO2 reduction performance [J]. Inorganic Chemicals Industry, 2023, 55(8): 45-50. |
| [7] | LAN Yinghua, CHEN Yanmei, MA Ruixiao, ZHANG Yanhui. Preparation and photocatalytic performance of Ce-Ti oxide-attapulgite composites [J]. Inorganic Chemicals Industry, 2023, 55(4): 133-140. |
| [8] | CHEN Zhangxu, FU Minglian, ZHU Danchen, ZHENG Bingyun. Preparation of carbon/graphite carbon nitride composites and their methylene blue removal performance [J]. Inorganic Chemicals Industry, 2023, 55(3): 134-139. |
| [9] | QIU Xiaokui, ZHANG Ruofan, WANG Xiaoyan, WANG Hailong, ZHANG Qixue, WAN Chao, XU Lixin. Research on bamboo shavings carbon supported ruthenium catalysts for hydrogen generation from photocatalytic hydrolysis of ammonia borane [J]. Inorganic Chemicals Industry, 2023, 55(10): 153-158. |
| [10] | ZHANG Zhe,LIAO Mingyu,CHEN Ming,YU Shanshan,ZHOU Kangdi,LI Jiachun,ZHANG Linfeng,WU Huadong,GUO Jia. Application of CeO2-ZnO/KIT-6 catalyst in photocatalytic adsorption desulfurization [J]. Inorganic Chemicals Industry, 2022, 54(9): 143-149. |
| [11] | TONG Haijuan,LI Siqi,FAN Fangfang,ZUO Weiyuan,SHI Bingfang. Facile synthesis of bismuth oxychloride and its photocatalytic degradation performance of p-nitrophenol [J]. Inorganic Chemicals Industry, 2022, 54(9): 157-162. |
| [12] | YANG Wenbo,WU Pan,HE Jian,LIU Changjun,JIANG Wei. Study on effect of heating rate on structure-activity of g-C3N4 photocatalyst by pyrolysis of urea [J]. Inorganic Chemicals Industry, 2022, 54(4): 169-174. |
| [13] | LIANG Fang,SHI Fanian. Research progress on synthesis of bismuth based bimetallic photocatalyst and degradation of organic pollutants [J]. Inorganic Chemicals Industry, 2022, 54(4): 61-68. |
| [14] | BAN Changsheng,LI Jun,JIN Yang,CHEN Ming,ZUO Longtao. Study on preparation of g-C3N4/g-C3N4 homojunction by supramolecular precursor and its photocatalytic property [J]. Inorganic Chemicals Industry, 2022, 54(3): 125-131. |
| [15] | CHEN Jianjun,QIAO Yan,LIU Zixian,PENG Huanhuan,SONG Jialin,GAO Yan,LI Yongyu. Preparation of Au-supported porous g-C3N4 nanosheets for photocatalytic H2 evolution performance under visible-light [J]. Inorganic Chemicals Industry, 2022, 54(3): 132-136. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||