Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (1): 97-101.doi: 10.11962/1006-4990.2020-0083
Previous Articles Next Articles
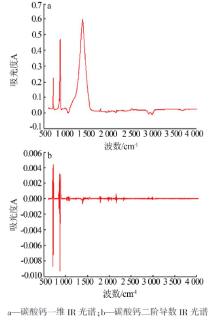
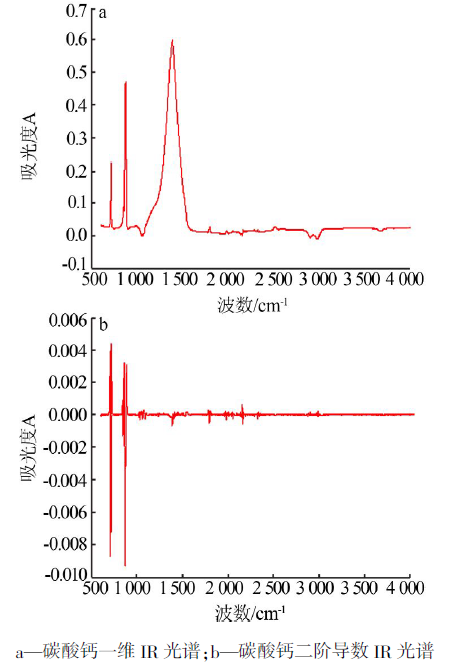
Study on third-step infrared spectroscopy of calcium carbonate
Zhang Bin( ),Xiao Xiao,Han Yunjiao,Zhang Yuanyuan,Yu Hongwei
),Xiao Xiao,Han Yunjiao,Zhang Yuanyuan,Yu Hongwei
- College of Chemical Technology,Shijiazhuang University,Shijiazhuang 050035,China
-
Received:2020-07-27Online:2021-01-10Published:2021-01-08 -
Contact:Zhang Yuanyuan,Yu Hongwei E-mail:zhyy701@163.com
CLC Number:
Cite this article
Zhang Bin,Xiao Xiao,Han Yunjiao,Zhang Yuanyuan,Yu Hongwei. Study on third-step infrared spectroscopy of calcium carbonate[J]. Inorganic Chemicals Industry, 2021, 53(1): 97-101.
share this article
"
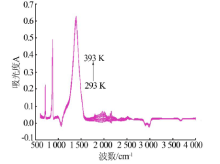
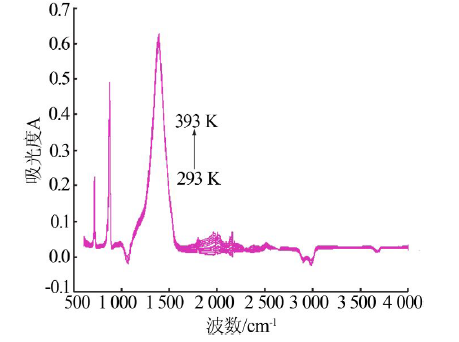
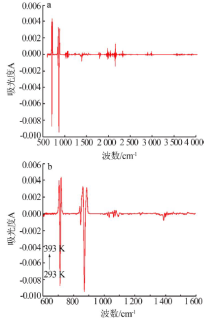
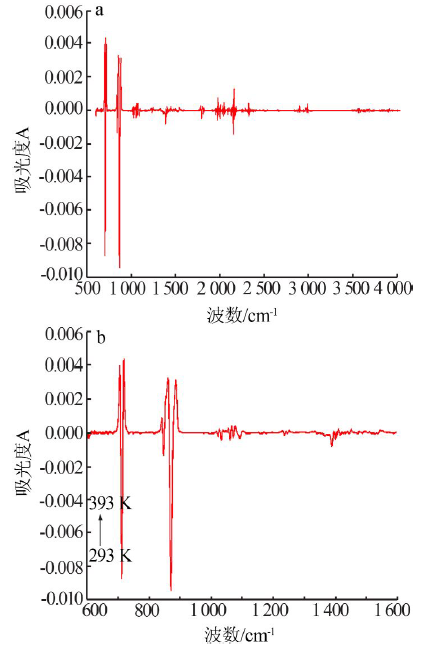
| 测量 温度/K | νC=O-碳酸钙+rC-O-碳酸钙/ cm-1(A) | νC=O-碳酸钙/ cm-1(A) | νasC-O-碳酸钙/ cm-1(A) | νsC-O-碳酸钙/ cm-1(A) | βC-O-碳酸钙/ cm-1(A) | βC-O-碳酸钙/ cm-1(A) | rC-O-碳酸钙/ cm-1(A) |
|---|---|---|---|---|---|---|---|
| 293 | 2 512.0(0.02) | 1 795.3(0.02) | 1 388.0(0.59) | 1 071.0(-) | 871.0(0.47) | 847.9(0.08) | 712.1(0.22) |
| 303 | 2 512.1(0.02) | 1 795.3(0.03) | 1 388.1(0.60) | 1 070.9(-) | 871.0(0.47) | 847.8(0.09) | 712.1(0.22) |
| 313 | 2 512.9(0.02) | 1 795.4(0.03) | 1 388.2(0.60) | 1 070.8(-) | 870.9(0.47) | 847.8(0.09) | 712.1(0.22) |
| 323 | 2 511.8(0.03) | 1 795.4(0.03) | 1 388.1(0.60) | 1 070.8(-) | 870.9(0.47) | 847.8(0.09) | 712.1(0.22) |
| 333 | 2 512.5(0.03) | 1 795.6(0.03) | 1 388.1(0.60) | 1 070.8(-) | 870.8(0.47) | 847.7(0.09) | 712.1(0.22) |
| 343 | 2 512.1(0.03) | 1 795.5(0.03) | 1 388.1(0.60) | 1 070.8(-) | 870.8(0.47) | 847.7(0.09) | 712.1(0.22) |
| 353 | 2 511.2(0.03) | 1 795.6(0.04) | 1 388.1(0.61) | 1 070.8(-) | 870.7(0.48) | 847.6(0.09) | 712.1(0.22) |
| 363 | 2 511.4(0.03) | 1 795.4(0.04) | 1 388.0(0.61) | 1 070.9(-) | 870.7(0.48) | 847.6(0.09) | 712.1(0.22) |
| 373 | 2 511.6(0.03) | 1 795.6(0.04) | 1 387.9(0.61) | 1 070.8(-) | 870.6(0.48) | 847.5(0.10) | 712.1(0.22) |
| 383 | 2 511.2(0.03) | 1 795.5(0.05) | 1 387.9(0.62) | 1 070.9(-) | 870.6(0.48) | 847.5(0.10) | 712.1(0.22) |
| 393 | 2 510.4(0.04) | 1 795.5(0.05) | 1 387.9(0.62) | 1 070.8(-) | 870.5(0.49) | 847.4(0.10) | 712.0(0.22) |
"
| 测量 温度/K | νC=O-碳酸钙/ cm-1 | νasC-O-碳酸钙/ cm-1 | νsC-O-碳酸钙/ cm-1 | βC-O-碳酸钙/ cm-1 | βC-O-碳酸钙/ cm-1 | rC-O-碳酸钙/ cm-1 |
|---|---|---|---|---|---|---|
| 293 | 1 795.7 | 1 388.8 | 1 071.5 | 871.1 | 847.1 | 712.1 |
| 303 | 1 795.7 | 1 388.8 | 1 071.5 | 871.0 | 847.1 | 712.1 |
| 313 | 1 795.6 | 1 388.7 | 1 071.6 | 871.0 | 847.0 | 712.1 |
| 323 | 1 795.5 | 1 388.7 | 1 071.6 | 871.0 | 847.0 | 712.1 |
| 333 | 1 795.5 | 1 388.6 | 1 071.6 | 870.9 | 846.9 | 712.1 |
| 343 | 1 795.5 | 1 388.6 | 1 071.5 | 870.9 | 846.9 | 712.1 |
| 353 | 1 795.4 | 1 388.6 | 1 071.6 | 870.8 | 846.8 | 712.1 |
| 363 | 1 795.4 | 1 388.5 | 1 071.6 | 870.8 | 846.8 | 712.1 |
| 373 | 1 795.4 | 1 388.5 | 1 071.6 | 870.7 | 846.7 | 712.1 |
| 383 | 1 795.3 | 1 388.5 | 1 071.6 | 870.6 | 846.7 | 712.1 |
| 393 | 1 795.3 | 1 388.4 | 1 071.5 | 870.6 | 846.6 | 712.1 |
"
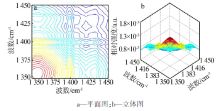
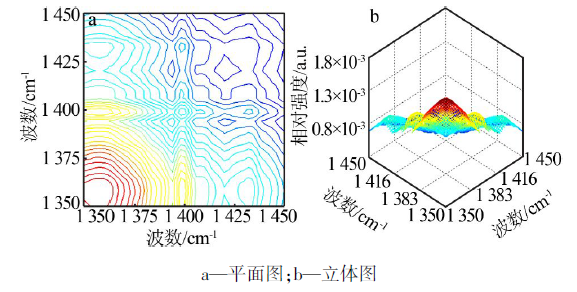
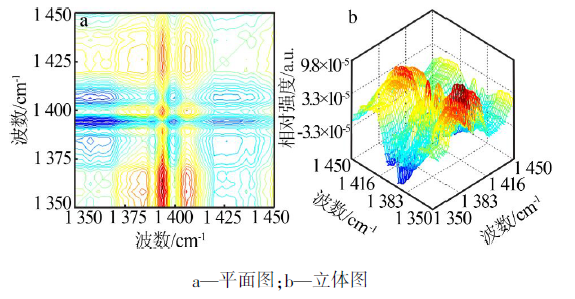
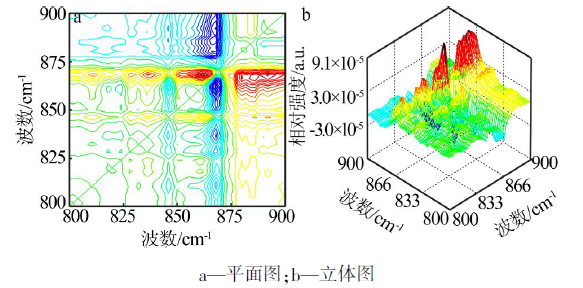
| ν1,ν2 /cm-1 | Ф(ν1,ν2) | Ψ(ν1,ν2) | 碳酸钙νasC-O-碳酸钙-二维的 2D-IR光谱数据及解释 |
|---|---|---|---|
| 1 388,1 393 | + | - | 1 393 cm-1(νasC-O-3-碳酸钙-二维)> 1 388 cm-1(νasC-O-4-碳酸钙-二维) |
| 1 388,1 398 | + | + | 1 388 cm-1(νasC-O-4-碳酸钙-二维)> 1 398 cm-1(νasC-O-2-碳酸钙-二维) |
| 1 388,1 407 | + | - | 1 407 cm-1(νasC-O-1-碳酸钙-二维)> 1 388 cm-1(νasC-O-4-碳酸钙-二维) |
| 1 393,1 398 | + | + | 1 393 cm-1(νasC-O-3-碳酸钙-二维)> 1 398 cm-1(νasC-O-2-碳酸钙-二维) |
| 1 393,1 407 | + | - | 1 407 cm-1(νasC-O-1-碳酸钙-二维)> 1 393 cm-1(νasC-O-3-碳酸钙-二维) |
| 1 398,1 407 | + | - | 1 407 cm-1(νasC-O-1-碳酸钙-二维)> 1 398 cm-1(νasC-O-2-碳酸钙-二维) |
"
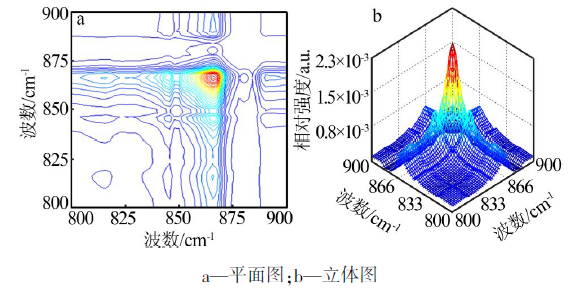
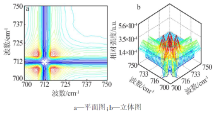
| ν1,ν2/cm-1 | Ф(ν1,ν2) | Ψ(ν1,ν2) | 碳酸钙βC-O-碳酸钙-二维 的 2D-IR光谱数据及解释 |
|---|---|---|---|
| 835,845 | + | + | 835 cm-1(βC-O-4-碳酸钙-二维)> 845 cm-1(βC-O-3-碳酸钙-二维) |
| 835,861 | + | + | 835 cm-1(βC-O-4-碳酸钙-二维)> 861 cm-1(βC-O-2-碳酸钙-二维) |
| 835,868 | + | + | 835 cm-1(βC-O-4-碳酸钙-二维)> 868 cm-1(βC-O-1-碳酸钙-二维) |
| 845,861 | + | - | 861 cm-1(βC-O-2-碳酸钙-二维)> 845 cm-1(βC-O-3-碳酸钙-二维) |
| 845,868 | + | + | 845 cm-1(βC-O-3-碳酸钙-二维)> 868 cm-1(βC-O-1-碳酸钙-二维) |
| 861,868 | + | + | 861 cm-1(βC-O-2-碳酸钙-二维)> 868 cm-1(βC-O-1-碳酸钙-二维) |
"
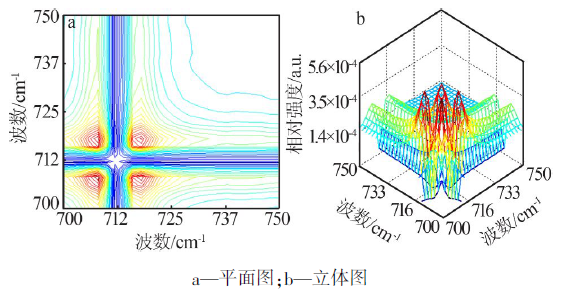
| ν1,ν2/cm-1 | Ф(ν1,ν2) | Ψ(ν1,ν2) | 碳酸钙 rC-O-碳酸钙-二维的2D-IR 光谱数据及解释 |
|---|---|---|---|
| 702,708 | + | + | 702 cm-1(rC-O-4-碳酸钙-二维)> 708 cm-1(rC-O-3-碳酸钙-二维) |
| 702,716 | + | + | 702 cm-1(rC-O-4-碳酸钙-二维)> 716 cm-1(rC-O-2-碳酸钙-二维) |
| 702,726 | + | + | 702 cm-1(rC-O-4-碳酸钙-二维)> 726 cm-1(rC-O-1-碳酸钙-二维) |
| 708,716 | + | + | 708 cm-1(rC-O-3-碳酸钙-二维)> 716 cm-1(rC-O-2-碳酸钙-二维) |
| 708,726 | + | - | 726 cm-1(rC-O-1-碳酸钙-二维)> 708 cm-1(rC-O-3-碳酸钙-二维) |
| 716,726 | + | - | 726 cm-1(rC-O-1-碳酸钙-二维)> 716 cm-1(rC-O-2-碳酸钙-二维) |
| [1] | 谭婷婷, 仲剑初. 球形碳酸钙的控制合成研究[J]. 无机盐工业, 2019,51(12):30-34. |
| [2] | 苗钧魁, 祝春英, 于跃芹, 等. 碳酸钙作为褐藻酸钠钙化剂的应用研究[J]. 无机盐工业, 2018,50(12):60-63. |
| [3] | 王琦, 郭德琪. 轻质碳酸钙制备工艺参数研究[J]. 无机盐工业, 2018,50(3):43-45. |
| [4] | 解爱莉, 曹林. 碳酸钙维生素 D3 咀嚼片的制备工艺[J]. 黑龙江科技信息, 2014(1):6. |
| [5] | 刘杏念, 王凌, 李斌, 等. 重质碳酸钙稳定的o/w型pickering乳液及其粒度效应研究[J]. 食品与机械, 2019,35(4):20-25,112. |
| [6] | 王跃猛, 刘安军, 李鑫, 等. 姜精油对明胶-CaCO3可食膜理化及抑菌特性影响的研究[J]. 现代食品科技, 2015(2):57-62,127. |
| [7] | 汤燕明, 孙丽霞, 郑燕宁, 等. 氮掺杂多孔碳材料固定化木瓜蛋白酶研究[J]. 添加剂与调味品, 2019,44(8):261-267. |
| [8] | 李雪莲, 杨丽, 赵梓辰, 等. 碳酸钙类矿物药红外光谱比较研究[J]. 时珍国医国药, 2014,25(10):2418-2419. |
| [9] | 杜成智, 邓家刚, 侯小涛, 等. 贝壳类中药碳酸钙含量测定及红外光谱比较[J]. 湖北农业科学, 2018,57(17):84-87. |
| [10] | 王振, 佟胜睿, 葛茂发. 大气不同粒径碳酸钙颗粒的红外光谱[J]. 光谱学与光谱分析, 2016,36(S1):135-136. |
| [11] | 尉念伦, 赵茉含, 陈丽云, 等. 聚苯乙烯变温红外光谱研究[J]. 纺织科学与工程学报, 2019,36(1):129-133. |
| [12] | 赵茉含, 高佳丽, 王欣, 等. PEEK变温红外光谱研究[J]. 纺织科学与工程学报, 2019,36(2):105-112. |
| [13] | 张铄, 周二鹏, 甄欢, 等. 东北亚麻纤维变温红外光谱研究[J]. 纺织科学与工程学报, 2018,35(3):94-98. |
| [14] | 甄欢, 周二鹏, 王小萌, 等. 山羊绒及绵羊毛变温红外光谱研究[J]. 纺织科学与工程学报, 2018,35(3):103-109. |
| [15] | 武玉洁, 王丽欣, 封卓帆, 等. 大米蛋白质酰胺Ⅲ带三级红外光谱研究[J]. 湖南文理学院学报:自然科学版, 2020,32(1):12-16. |
| [16] | 张文燕, 申澳, 郑雨倩, 等. 锦纶-66 νamide-Ⅰ和 νamide-Ⅱ三级红外光谱[J]. 纺织科学与工程学报, 2018,35(4):126-132. |
| [17] | 赵茉含, 张莹, 王慧慧, 等. 小米蛋白质三级红外光谱研究[J]. 伊犁师范学院学报:自然科学版, 2019,13(3):38-43. |
| [18] | 于宏伟, 赵婷婷, 杨晓雪, 等. 杨絮纤维及柳絮纤维的三级红外光谱研究[J]. 成都纺织高等专科学校学报, 2017,34(3):17-24. |
| [1] | WANG Ruting, ZHAO Xiaorong, HUANG Xuquan, WANG Haojie, XUE Fei, CAI Jiawei. Research on preparation and early performance of mixed phase phosphogypsum-based cementing materials [J]. Inorganic Chemicals Industry, 2024, 56(3): 98-104. |
| [2] | LI Qiang, YOU Xiaomin, SHE Xuefeng, JIANG Zeyi, XUE Qingguo, WANG Jingsong. Effect of calcination temperature and carbon structure on compressive strength of CaO-containing carbon pellets [J]. Inorganic Chemicals Industry, 2023, 55(9): 43-49. |
| [3] | CHEN Yanmeng, MO Huiling, ZHONG Jiamei, YANG Pengfei, LAN Junfeng. Study on preparation process of porous calcium carbonate by carbonization [J]. Inorganic Chemicals Industry, 2023, 55(8): 102-108. |
| [4] | YU Hongchao, ZHANG Mengmeng, JIN Tianxiang. Research progress of microstructure and crystal surface effect of Ag3PO4 photocatalysts [J]. Inorganic Chemicals Industry, 2023, 55(8): 13-20. |
| [5] | ZHANG Jinjun, GUO Linlin, LIU Bojing, FENG Shuang, SHI Qi. Study on preparation of needle-like shaped CaCO3 from calcium carbide slag [J]. Inorganic Chemicals Industry, 2023, 55(7): 103-108. |
| [6] | HE Yanfang, ZHAO Lüxuan, FENG Yuan, WU Kaiqing, LIU Bingyan, HUANG Junkai. Countermeasures and suggestions for high-quality development of calcium carbonate industry and ecological environment protection in Guangxi [J]. Inorganic Chemicals Industry, 2023, 55(7): 25-31. |
| [7] | QU Xiaoyuan, ZHENG Qiang, FAN Yuanyang, LIU Haili, DENG Xiaoyang, LI Xue. Study on preparation of large cube calcium carbonate by secondary carbonization of dolomite [J]. Inorganic Chemicals Industry, 2023, 55(7): 58-64. |
| [8] | XU Li, ZHANG Qiang. Experimental study on properties of iron tailings powder cement-based materials [J]. Inorganic Chemicals Industry, 2023, 55(6): 116-123. |
| [9] | LUO Zhibo, WANG Huaiyou, WANG Min, DU Baoqiang. Effect of purity on thermo-physical properties of 60% NaNO3-40% KNO3 binary molten salt [J]. Inorganic Chemicals Industry, 2023, 55(6): 43-49. |
| [10] | LI Zhiqiang, GUO Zhenghua, HE Yuru. Effect of Zr/Mn element substitution and annealing treatment on electrochemical properties of hydrogen storage alloys for automotive batteries [J]. Inorganic Chemicals Industry, 2023, 55(6): 78-84. |
| [11] | JIA Yuhong, HU Zhongpan, WANG Kunyuan, HAN Jingfeng, WEI Yingxu, LIU Zhongmin. Co anchored on silanol nests of S-1 zeolite for propane dehydrogenation to propylene [J]. Inorganic Chemicals Industry, 2023, 55(5): 121-127. |
| [12] | WEI Fengdan, ZHOU Huan, XIA Panping, ZHAO Yun. Boron species transformation and distribution law of Mg(BO2)2decomposed in LiCl aqueous solution [J]. Inorganic Chemicals Industry, 2023, 55(4): 45-53. |
| [13] | MA Xue, ZHOU Yuan, HUANG Chao, ZHU Xiaping. Study on preparation process of high purity calcium citrate from Zigong underground yellow brine [J]. Inorganic Chemicals Industry, 2023, 55(4): 60-64. |
| [14] | LI Liangrong, YANG Xiaozhe, CHEN Chuxin, LIU Yan, ZHANG Mengling, DING Yonghong. Research progress of photocatalytic water splitting of semiconductor core-shell materials for hydrogen production [J]. Inorganic Chemicals Industry, 2023, 55(3): 10-20. |
| [15] | LI Peng, WANG Likun, MENG Qiuyan. Study on effect of α-hemihydrate gypsum on performance of cement mortar and its hydration mechanism [J]. Inorganic Chemicals Industry, 2023, 55(3): 98-103. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||