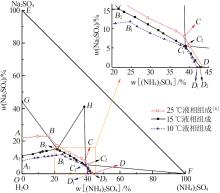
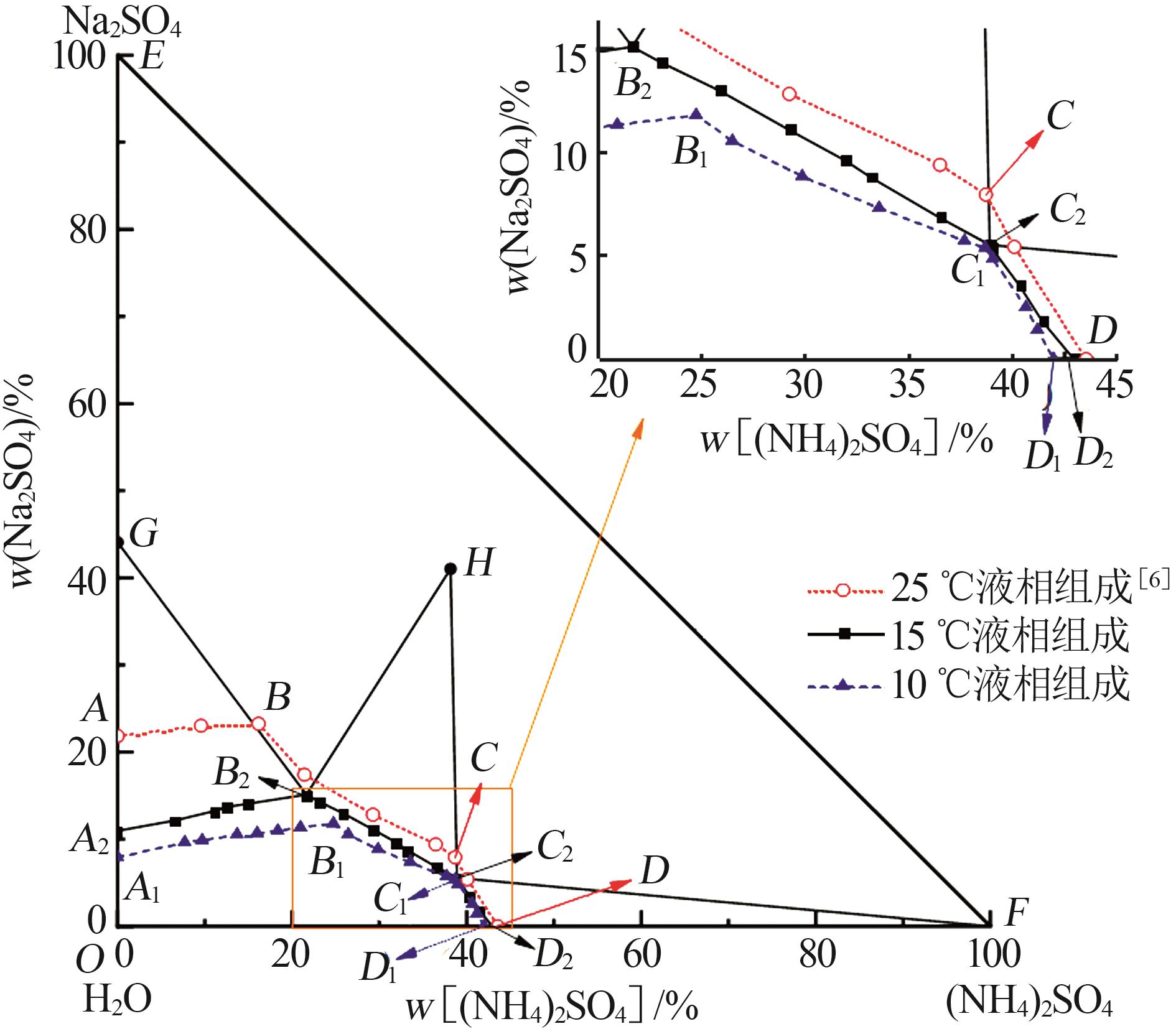
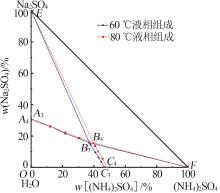
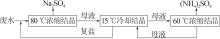
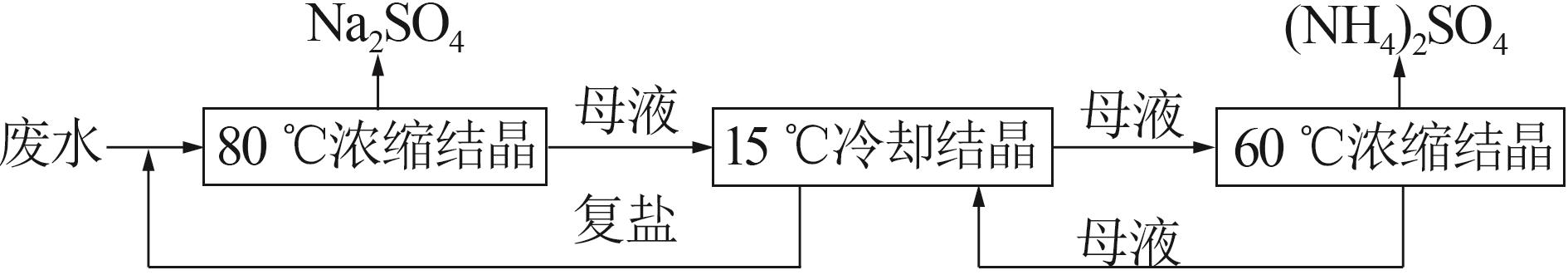
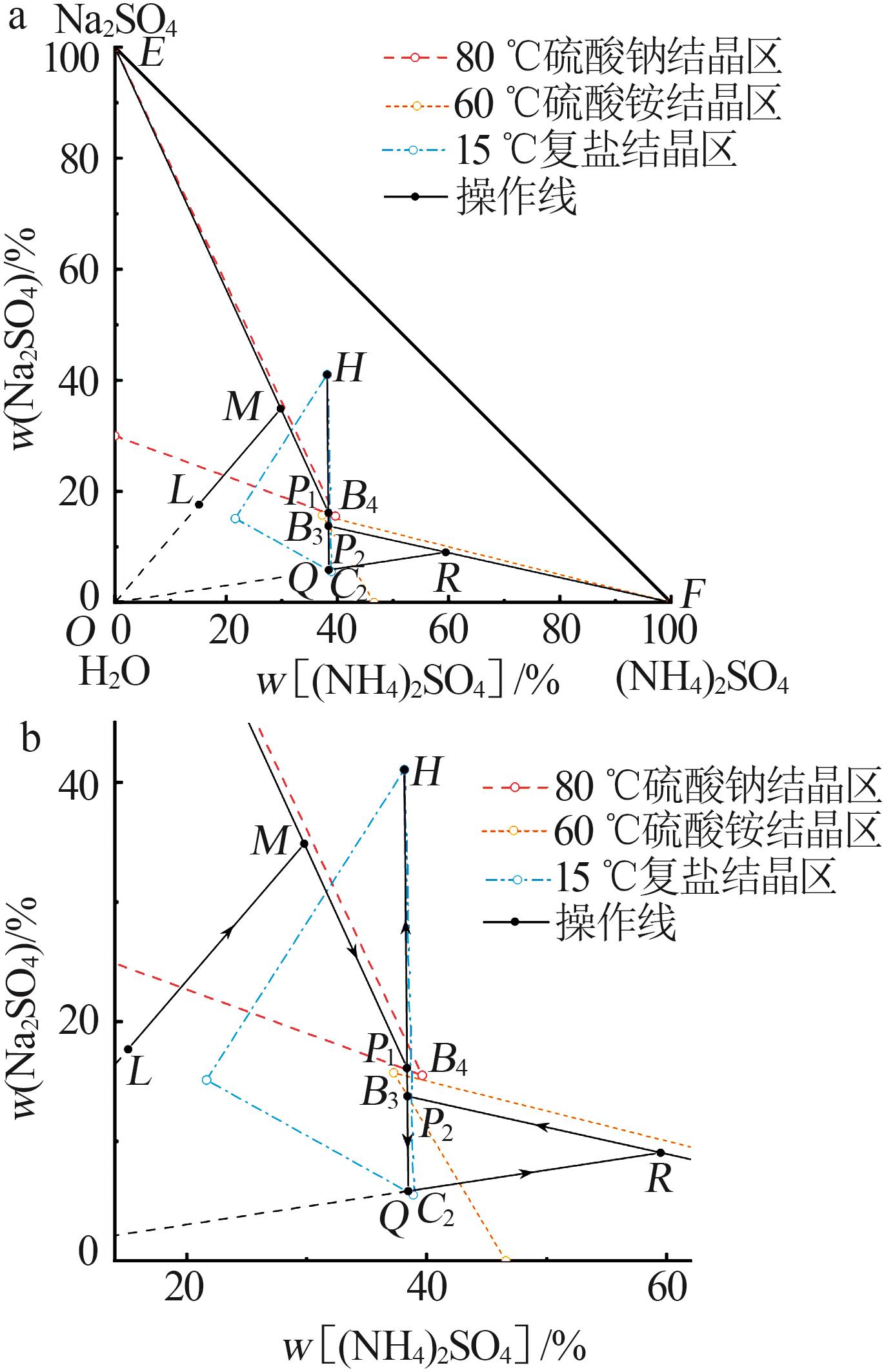
| [1] |
ZHOU Zhaoan, LI Jun, LIU Xiaowen, ZHOU Aiqing, MAO Anzhang.
Study on carbonization and purification process of high COD industrial waste salt
[J]. Inorganic Chemicals Industry, 2023, 55(9): 100-105.
|
| [2] |
WANG Song, WANG Jiawei, GOU Bibo, YANG Pan, HE Yue, YANG Chunyuan, WANG Haifeng.
Study on distribution law of magnesium in leaching solution of rhodochrosite by complex salt crystallization method
[J]. Inorganic Chemicals Industry, 2023, 55(4): 65-71.
|
| [3] |
PAN Sicheng,XU Hongbin,ZHANG Hongling,DONG Yuming,ZHANG Hongjun,LOU Taiping.
Study on preparation of metatitanic acid by hydrolysis of leaching solution of roasted mixture of red mud and ammonium sulfate
[J]. Inorganic Chemicals Industry, 2023, 55(2): 85-91.
|
| [4] |
WU Di, LI Laishi, WANG Junkai, WU Yusheng, WANG Yuzheng, LI Mingchun.
Study on decomposition process and thermal decomposition kinetics of ammonium sulfate
[J]. Inorganic Chemicals Industry, 2023, 55(10): 86-92.
|
| [5] |
WANG Bingjian,QI Daozheng,CHEN Deng.
Degradation processes and mechanism of tricalcium aluminate in different sulfate environment
[J]. Inorganic Chemicals Industry, 2022, 54(8): 119-124.
|
| [6] |
ZHENG Sanqiang,LI Xingbin,LUO Xingguo,WEI Chang,HUANG Xing,DENG Zhigan,LI Minting.
Production and control of water insoluble substance in sodium sulfate produced by NaCl-Na2SO4 co?production
[J]. Inorganic Chemicals Industry, 2022, 54(8): 90-95.
|
| [7] |
LIU Zhuang,AN Jimin,LI Yongjun,CHEN Xing,ZHAO Yigang,ZHAI Ruiguo.
Effect of impurity in alkaline washing oxidation process on crystal size of sodium sulfate
[J]. Inorganic Chemicals Industry, 2022, 54(4): 123-127.
|
| [8] |
YANG Han,DENG Fuli,DING Yao,LONG Bingwen.
Experimental study on comprehensive utilization of citric acid soluble phosphorus sludge
[J]. Inorganic Chemicals Industry, 2022, 54(12): 87-91.
|
| [9] |
QI Yuanhao,WU Jinxiu,LIU Zhaogang,HU Yanhong,FENG Fushan,LI Jianfei,WANG Xin,LIU Conglin.
Preparation and characterization of anhydrous calcium sulfate whisker
[J]. Inorganic Chemicals Industry, 2022, 54(10): 109-115.
|
| [10] |
Tian Haiying,Zhou Fu,Xu Chuan,Tu Mingjiang,Zhong Zhaozi,Liao Shiying,Li Shihong.
Application research of Li+,Na+,K+,SO42--H2O system phase diagram in MVR evaporation process
[J]. Inorganic Chemicals Industry, 2021, 53(8): 75-78.
|
| [11] |
Wang Yanfei,Jiao Jian,Jiang Shuwan,Xu Shijie.
Study on nucleation kinetics of sodium sulfate-water system based on inverse solubility
[J]. Inorganic Chemicals Industry, 2021, 53(10): 41-46.
|
| [12] |
Lei Shaocheng,Li Yulin.
Experimental study on salt separation of high salt wastewater from coal chemical HERO by DTNF nanofiltration membrane
[J]. Inorganic Chemicals Industry, 2020, 52(9): 84-87.
|
| [13] |
Zhang Bingbing,Ren Jianpo,Shen Zhihong,Jiang Zhiqiang.
Experimental study on tail gas absorption in synthesis of 2,3-pyridinedicarboxylic acid
[J]. Inorganic Chemicals Industry, 2020, 52(7): 74-76.
|
| [14] |
Liu Yuelong,Wang Linlin,Liu Gousheng.
Extraction of rubidium and cerium salts from lithium tail liquid of medium and low grade lithium clay by ammonium sulfate process
[J]. Inorganic Chemicals Industry, 2020, 52(11): 60-63.
|
| [15] |
Liao Enxin,Chen Lifang,Zhang Zeya,Bai Fengxia.
Experimental study on preparing potassium sulfate by sodium sulfate and potassium chloride
[J]. Inorganic Chemicals Industry, 2020, 52(10): 106-109.
|
 ), JI Lijun1(
), JI Lijun1( ), SHENG Yong2, CHEN Kui1, WU Yanyang1, WU Bin1
), SHENG Yong2, CHEN Kui1, WU Yanyang1, WU Bin1