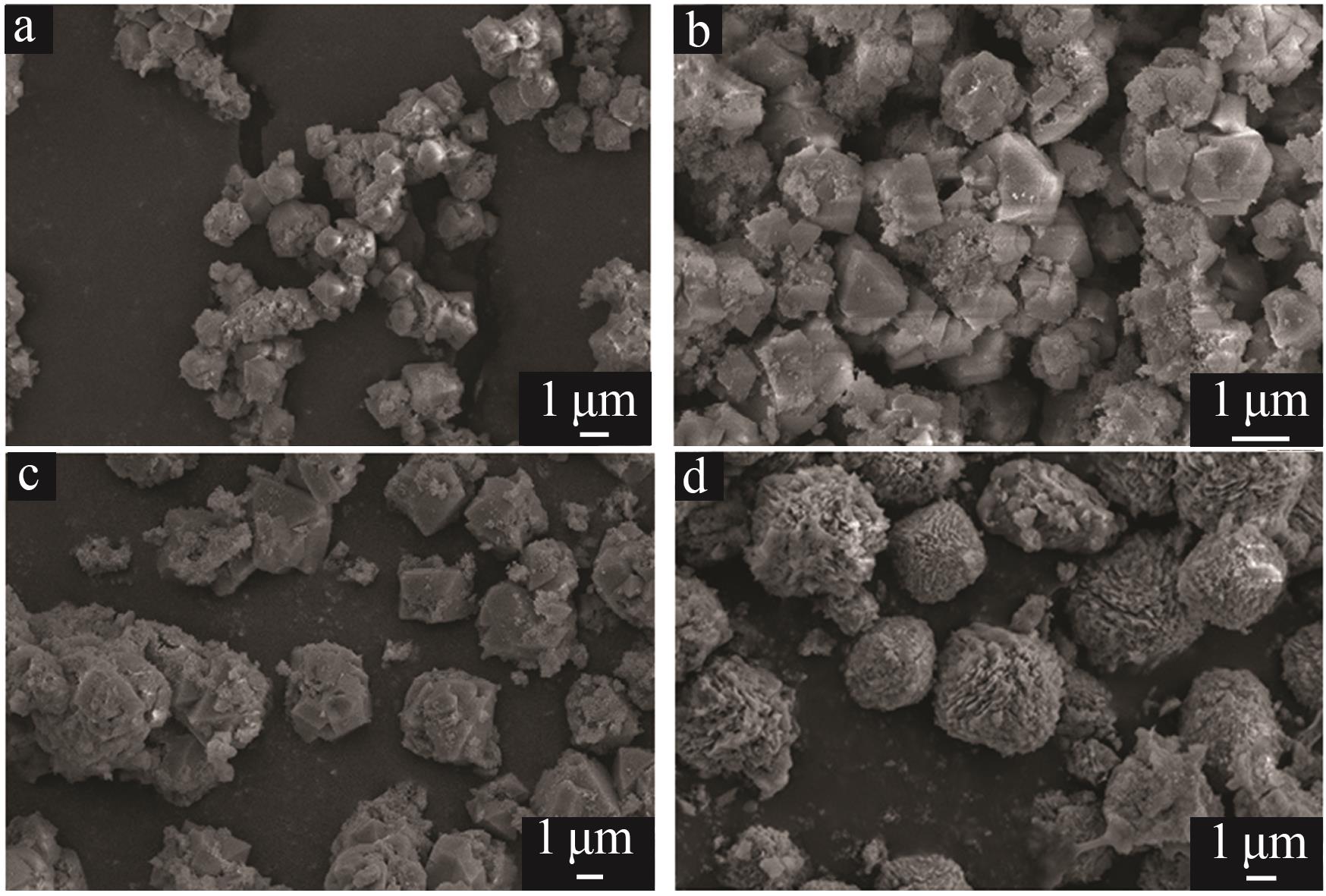
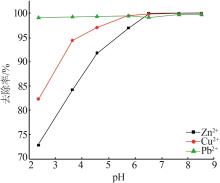
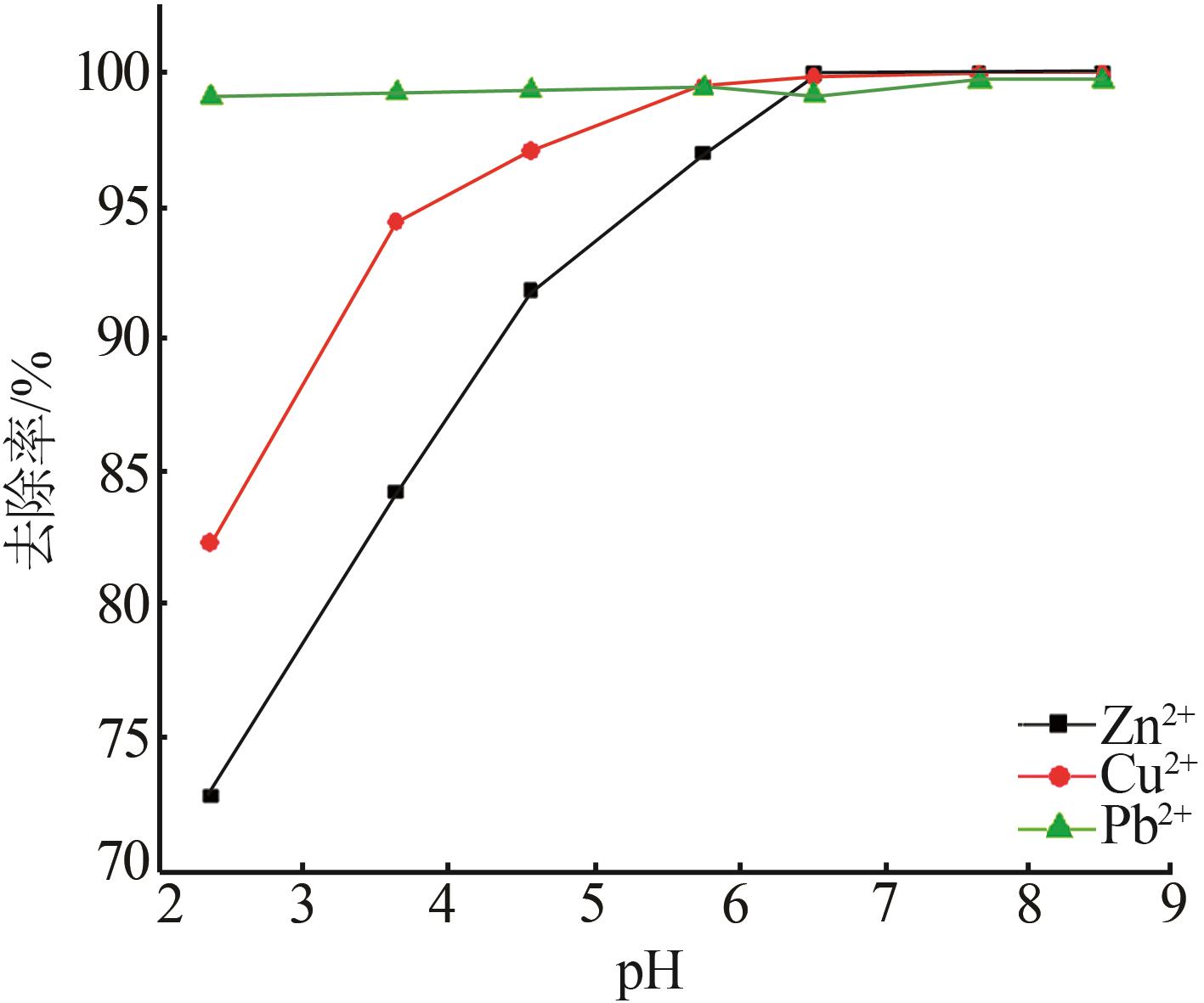
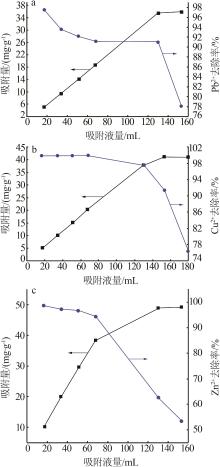
| 1 |
李超,王丽萍,郭昭华,等.粉煤灰提铝后尾渣合成13X分子筛及其对Pb2+吸附性能的研究[J].矿产保护与利用,2018(6):98-102.
|
|
LI Chao, WANG Liping, GUO Zhaohua,et al.Synthesis of 13X zeolite by fly ash acid residue and its adsorption performance on lead ions[J].Conservation and Utilization of Mineral Resources,2018(6):98-102.
|
| 2 |
李超,王丽萍,郭昭华,等.粉煤灰酸溶渣合成13X分子筛及其对铜离子吸附性能[J].无机盐工业,2018,50(9):63-66.
|
|
LI Chao, WANG Liping, GUO Zhaohua,et al.Synthesis of 13X zeolite by fly ash acid residue and its adsorption performance to copper ions[J].Inorganic Chemicals Industry,2018,50(9):63-66.
|
| 3 |
司玉成,吴涛.制备13X分子筛的优化实验研究[J].矿产综合利用,2022(4):157-161.
|
|
SI Yucheng, WU Tao.Research on optimization experiment of preparing 13X molecular sieve[J].Multipurpose Utilization of Mineral Resources,2022(4):157-161.
|
| 4 |
LIU Huidong.Conversion of harmful fly ash residue to zeolites:Innovative processes focusing on maximum activation,extraction,and utilization of aluminosilicate[J].ACS Omega,2022,7(23):20347-20356.
|
| 5 |
李超,王丽萍.选矿废水处理技术的研究进展[J].矿产保护与利用,2020,40(1):72-78.
|
|
LI Chao, WANG Liping.Research progress of mine wastewater treatment technology[J].Conservation and Utilization of Mineral Resources,2020,40(1):72-78.
|
| 6 |
SOE J T, KIM S S, LEE Yuri,et al.CO2 capture and Ca2+ exchange using zeolite A and 13X prepared from power plant fly ash[J].Bulletin of the Korean Chemical Society,2016,37(4):490-493.
|
| 7 |
MURUKUTTI M K, JENA H.Synthesis of nano-crystalline zeolite-A and zeolite-X from Indian coal fly ash,its characterization and performance evaluation for the removal of Cs+ and Sr2+ from simulated nuclear waste[J].Journal of Hazardous Materials,2022,423:127085.
|
| 8 |
陶红,徐国勋,马鸿文.13X分子筛处理重金属废水的试验研究[J].中国给水排水,2000,16(5):53-56.
|
|
TAO Hong, XU Guoxun, MA Hongwen.Experimental research on the treatment of wastewater containing heavy metals by 13X molecular sieve[J].China Water & Wastewater,2000,16(5):53-56.
|
| 9 |
朱彤,张翔宇,宋宝华,等.分子筛对重金属废水吸附性能的实验研究[J].无机盐工业,2012,44(1):49-51.
|
|
ZHU Tong, ZHANG Xiangyu, SONG Baohua,et al.Study on adsorption of heavy metals from wastewater by zeolite[J].Inorganic Chemicals Industry,2012,44(1):49-51.
|
| 10 |
孙秀云,马芳变,施筱堃,等.粉煤灰合成介孔分子筛SBA-15对Pb(Ⅱ)离子的吸附[J].中南大学学报(自然科学版),2014,45(11):4093-4099.
|
|
SUN Xiuyun, MA Fangbian, SHI Xiaokun,et al.Adsorption of Pb(Ⅱ) on mesoporous molecular sieve SBA-15 synthesized from fly ash[J].Journal of Central South University(Science and Technology),2014,45(11):4093-4099.
|
| 11 |
肖万,马鸿文,杨静,等.13X沸石对Ni2+吸附性能的实验研究[J].地球科学,2003,28(1):21-25.
|
|
XIAO Wan, MA Hongwen, YANG Jing,et al.Adsorption of 13X zeolite on Ni2+ in wastewater:An experimental study[J].Earth Science,2003,28(1):21-25.
|
| 12 |
王鲲鹏,李科,廖维,等.13X分子筛对水体中钒离子的吸附[J].环境工程学报,2016,10(11):6249-6254.
|
|
WANG Kunpeng, LI Ke, LIAO Wei,et al.Adsorption of vanadium on 13X molecular sieve in aqueous solution[J].Chinese Journal of Environmental Engineering,2016,10(11):6249-6254.
|
| 13 |
朱成斌,范先媛,龙云川,等.pH对13X分子筛去除Pb(Ⅱ)性能的影响研究[J].水处理技术,2021,47(1):43-48.
|
|
ZHU Chengbin, FAN Xianyuan, LONG Yunchuan,et al.Study on the effect of pH on the removal of Pb(Ⅱ) ions by 13X molecular sieves[J].China Industrial Economics,2021,47(1):43-48.
|
| 14 |
陶春光.淮南电厂粉煤灰制备分子筛及提取硅铝的研究[D].淮南:安徽理工大学,2019.
|
|
TAO Chunguang.Study on preparation of molecular sieve and extraction of silicon and aluminum from fly ash of Huainan power plant[D].Huainan:Anhui University of Science & Technology,2019.
|
| 15 |
陶春光,田冬,陈永红,等.粉煤灰尾渣碱融水热合成高性能13 X分子筛[J].硅酸盐通报,2019,38(3):622-626,633.
|
|
TAO Chunguang, TIAN Dong, CHEN Yonghong,et al.Fly ash tailings alkali melting hydrothermal synthesis high performance 13X molecular sieves[J].Bulletin of the Chinese Ceramic Society,2019,38(3):622-626,633.
|
| 16 |
竹涛,韩一伟,牛文风,等.粉煤灰制备13X分子筛及VOCs吸附性能研究[J].煤炭科学技术,2021,49(7):216-222.
|
|
ZHU Tao, HAN Yiwei, NIU Wenfeng,et al.Preparation of 13X zeolite by coal fly ash for adsorption of volatile organic compounds[J].Coal Science and Technology,2021,49(7):216-222.
|
| 17 |
LEE H J, KIM Y M, KWEON O S,et al.Structural and morphological transformation of NaX zeolite crystals at high temperatu-re[J].Journal of the European Ceramic Society,2007,27(2/3):561-564.
|
| 18 |
NEZAMZADEH-EJHIEH A, GHANBARI-MOBARAKEH Z.Heterogeneous photodegradation of 2,4-dichlorophenol using FeO doped onto nano-particles of zeolite P[J].Journal of Industrial and Engineering Chemistry,2015,21:668-676.
|
 ), WANG Liping(
), WANG Liping( ), DAI Yin, GAO Guimei, ZHANG Yunfeng, HONG Yu, XU Lijun, CUI Yongjie
), DAI Yin, GAO Guimei, ZHANG Yunfeng, HONG Yu, XU Lijun, CUI Yongjie