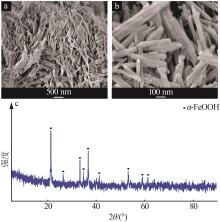
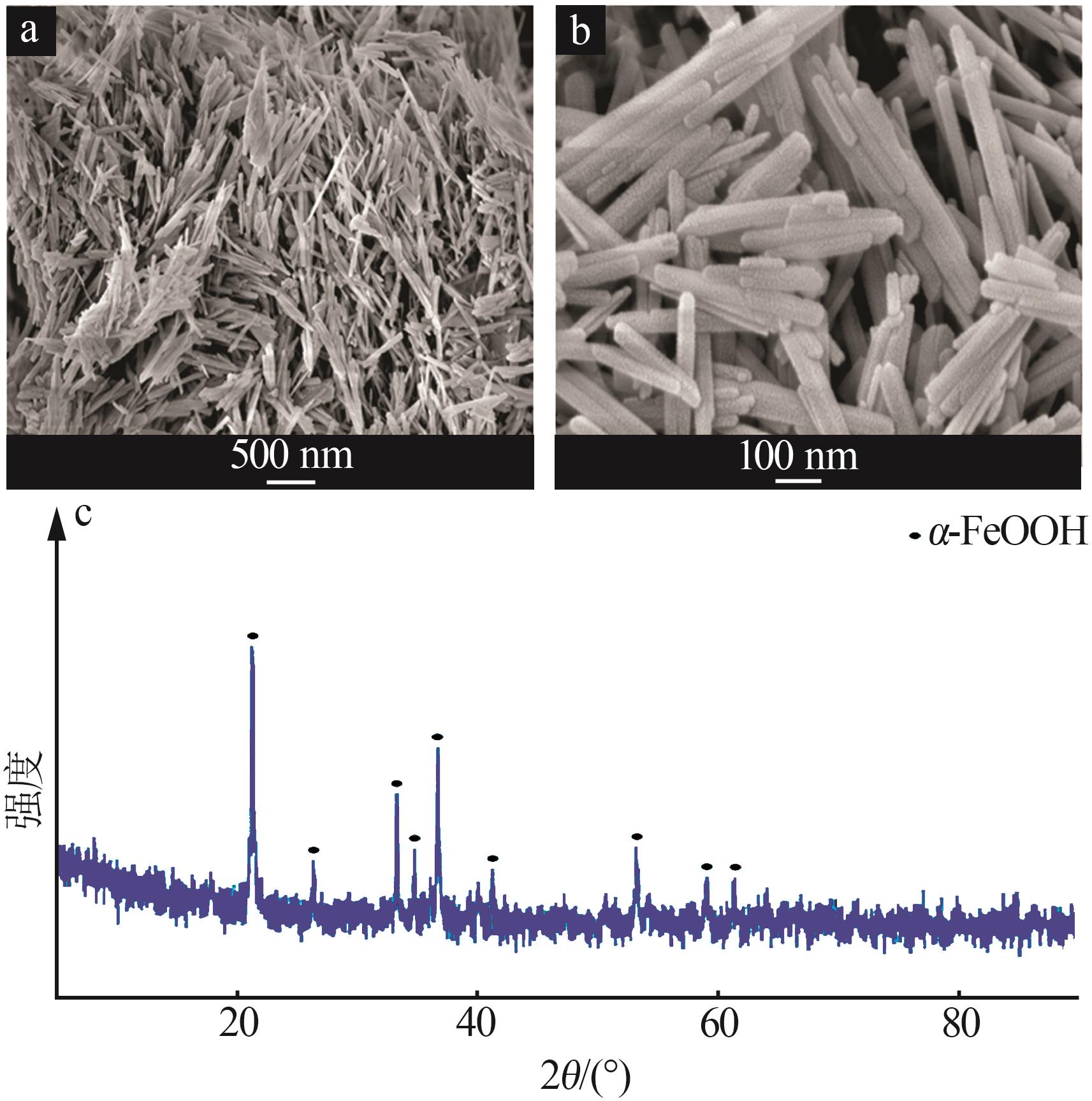
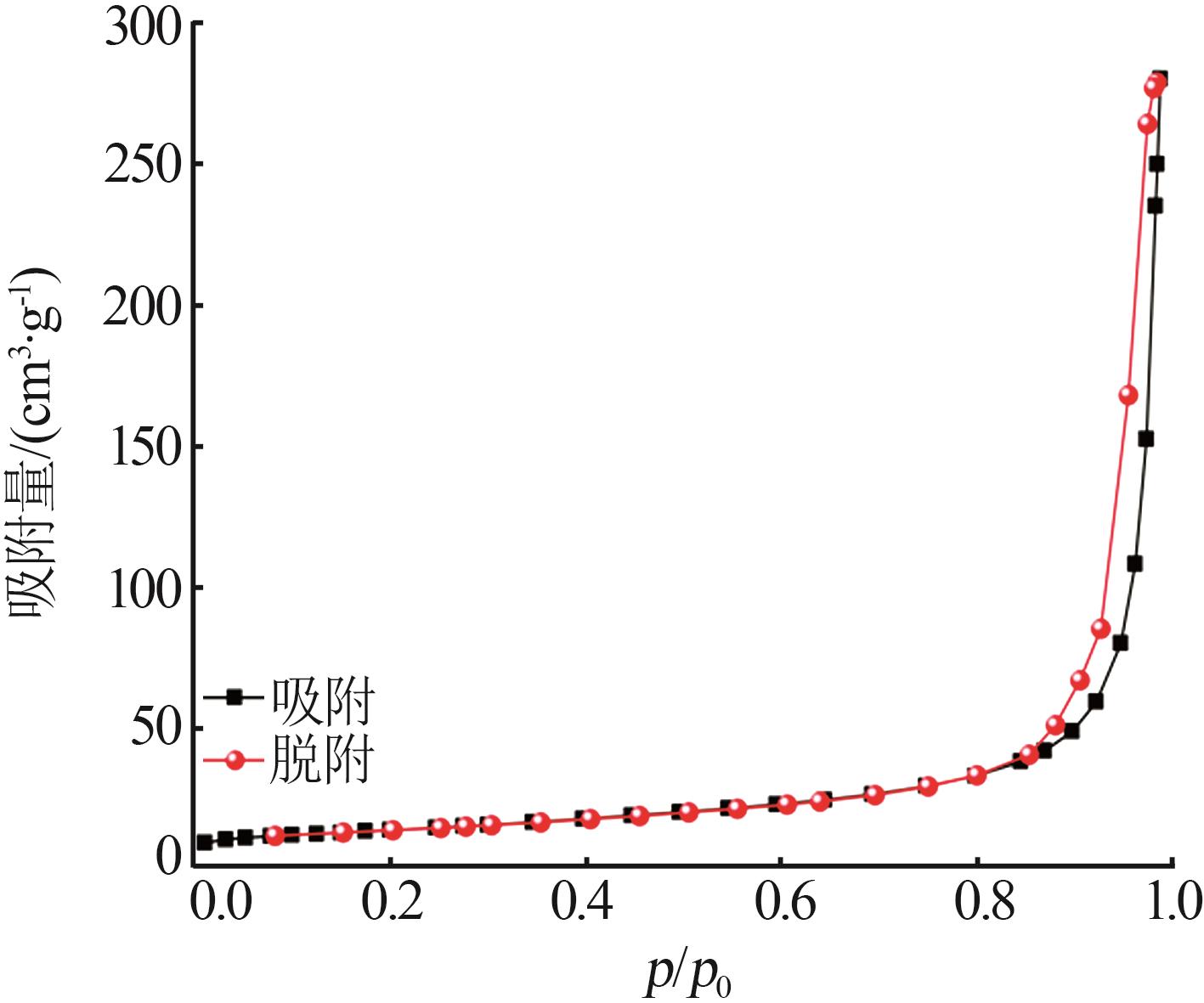
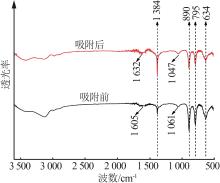
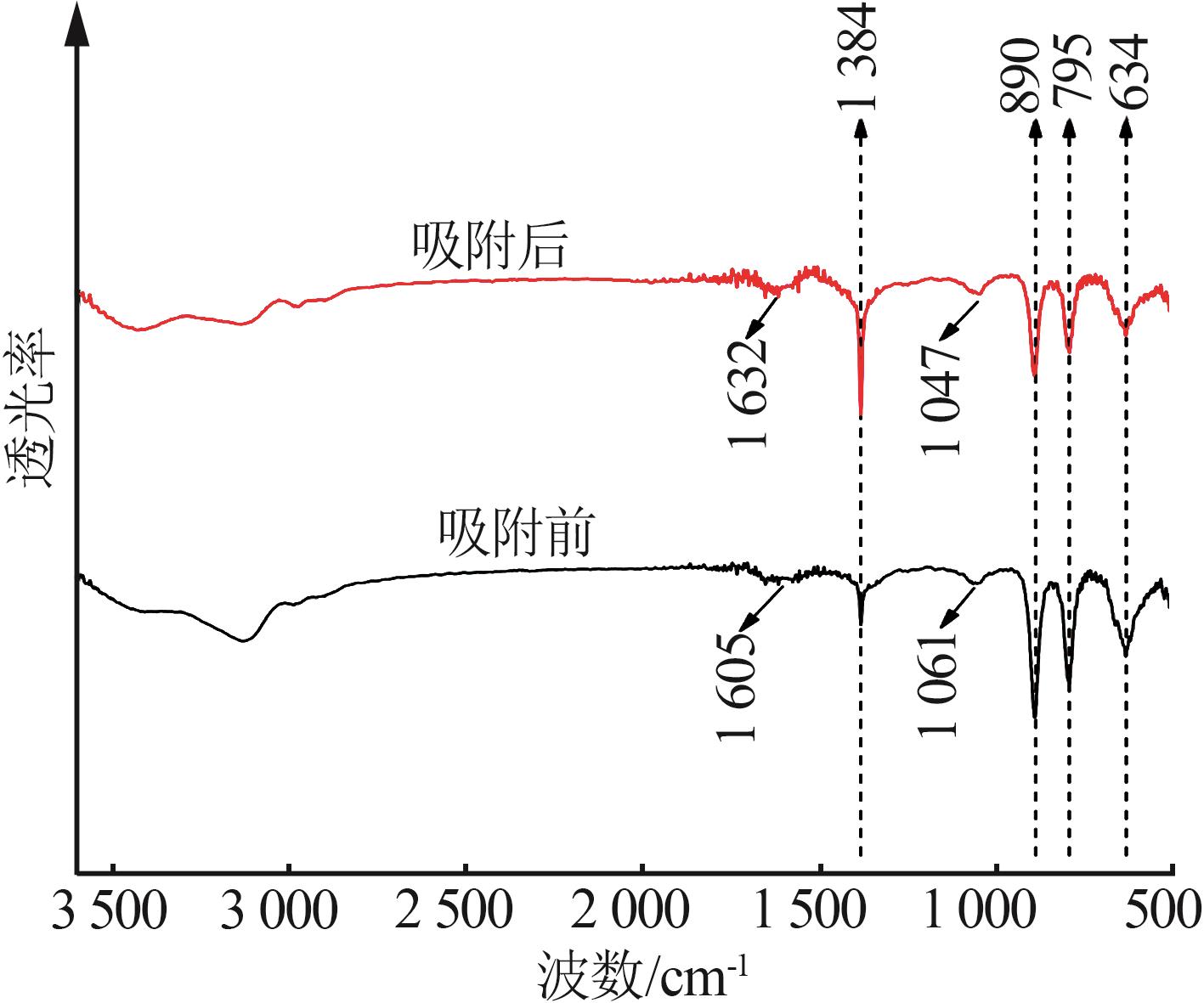
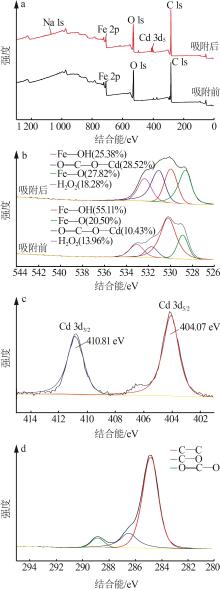
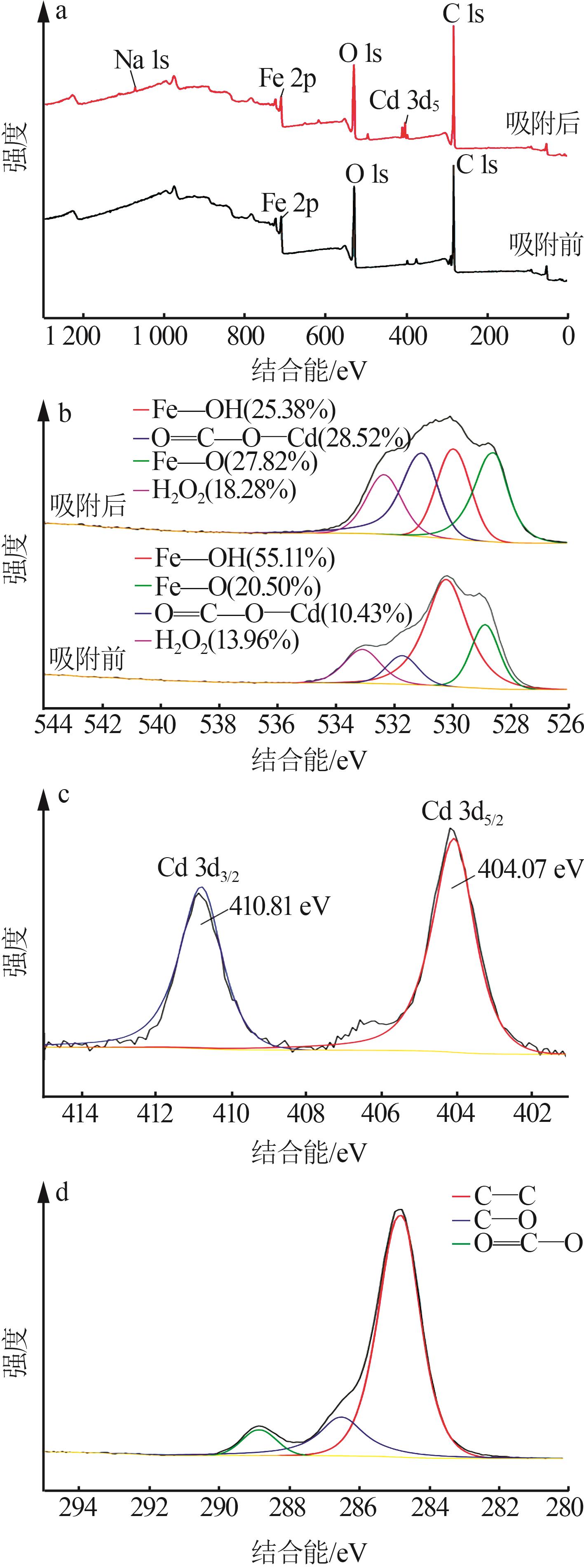
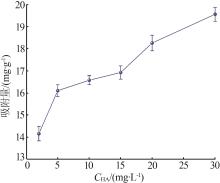
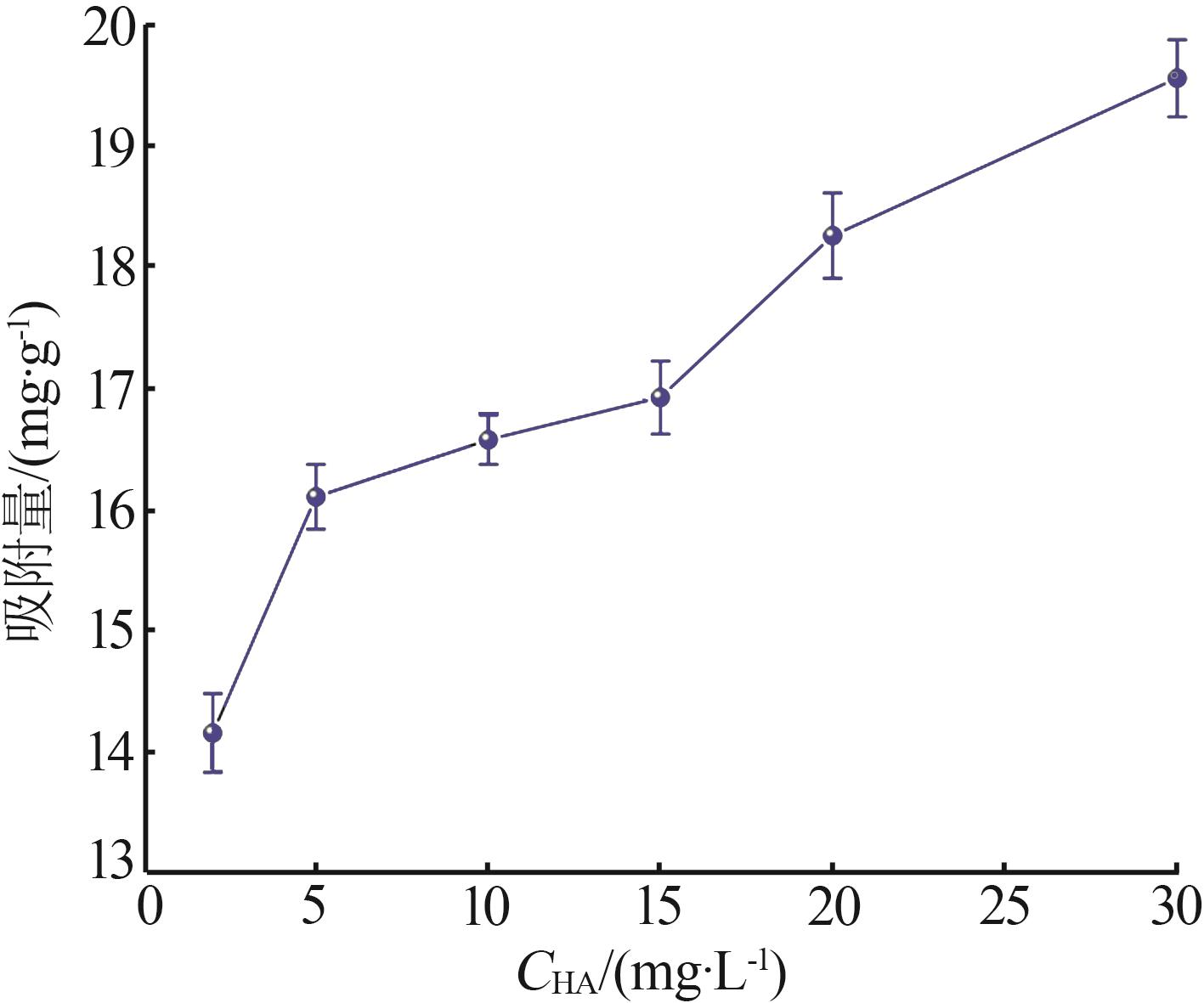
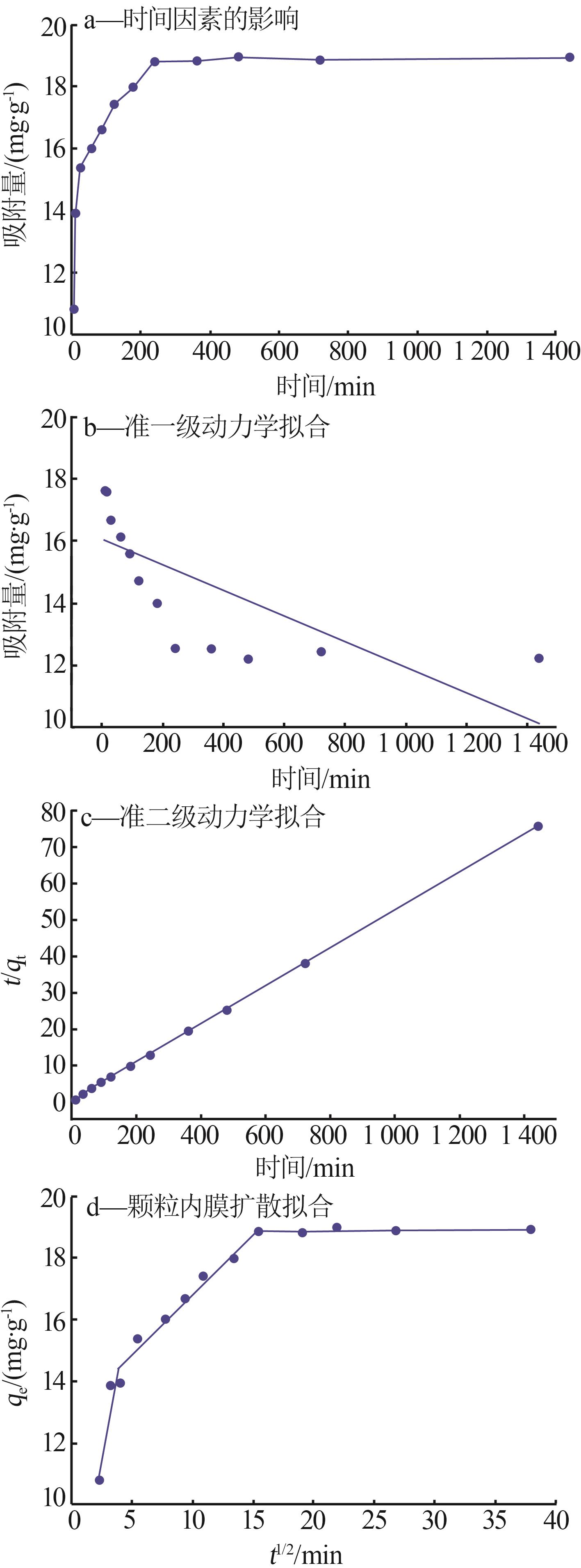
Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (8): 124-131.doi: 10.19964/j.issn.1006-4990.2022-0643
• Environment·Health·Safety • Previous Articles Next Articles
Study on adsorption of cadmium-humic acid by hydroxyiron oxide
TANG Yifu1( ), CAO Changchun1,2(
), CAO Changchun1,2( ), LÜ Peng1
), LÜ Peng1
- 1.College of Environmental Science and Engineering,Guilin University of Technology,Guilin 541004,China
2.Guangxi Collaborative Innovation Center for Water Pollution Control and Water Safety in Karst Areas,Guilin 541004,China
-
Received:2022-11-01Online:2023-08-10Published:2023-08-25 -
Contact:CAO Changchun E-mail:yvantang77@163.com;caochch@glite.edu.cn
CLC Number:
Cite this article
TANG Yifu, CAO Changchun, LÜ Peng. Study on adsorption of cadmium-humic acid by hydroxyiron oxide[J]. Inorganic Chemicals Industry, 2023, 55(8): 124-131.
share this article
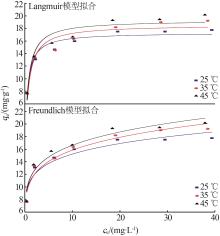
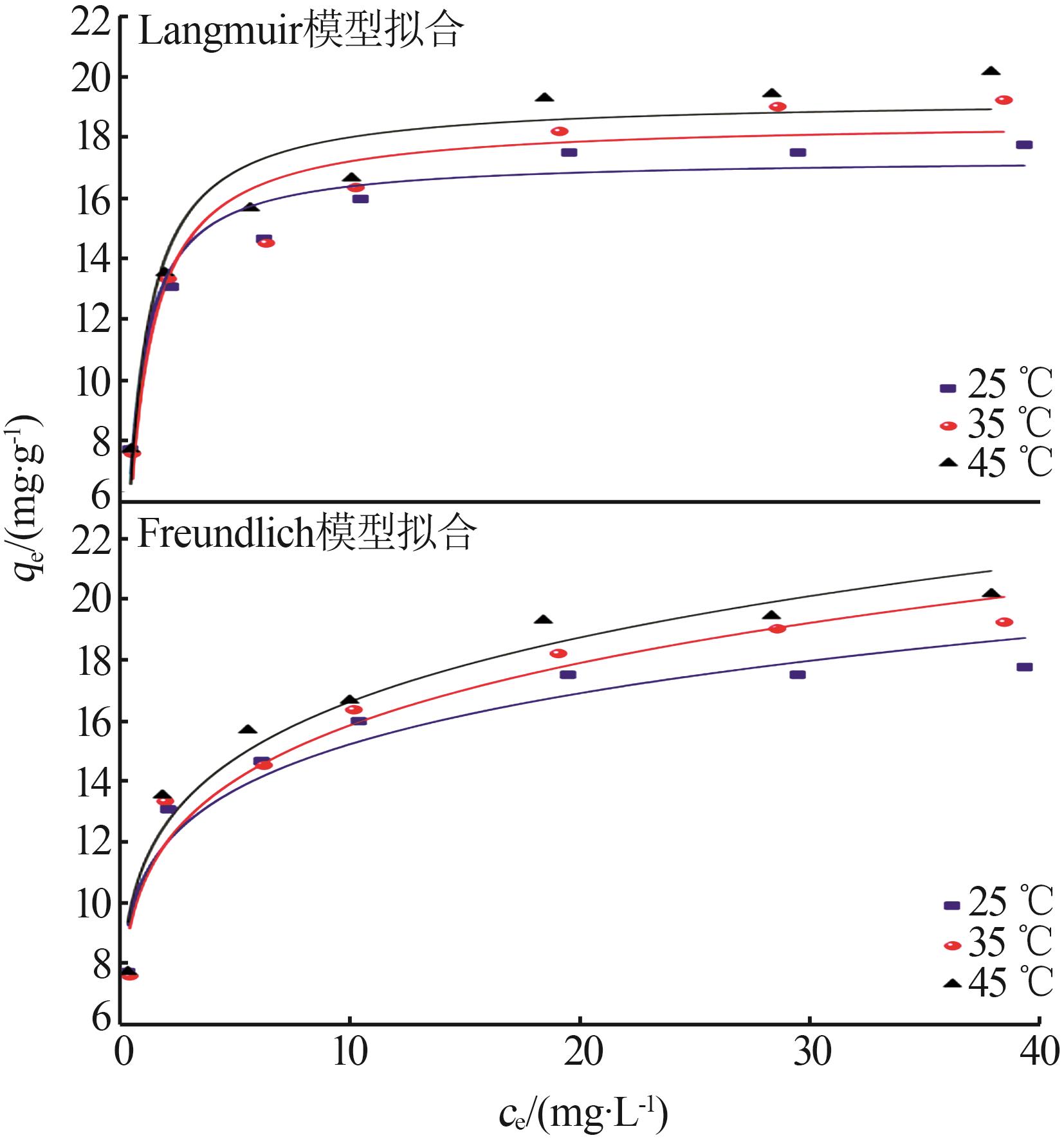
Table 1
Parameters for fitting kinetic model andparticle inner film diffusion model"
| 实验数值 | 准一级 动力学模型 | 准二级 动力学模型 | 颗粒内膜 扩散模型 |
|---|---|---|---|
qe= 18.79 mg/g | qe= 4.567 mg/g | qe= 20.686 mg/g | kdi1=2.017 mg·g-1·min0.5 kdi2=0.388 mg·g-1·min0.5 kdi3=0.006 mg·g-1·min0.5 |
k1= 0.001 mg/min | k2= 0.007 14 mg/min | Ci1=6.652 Ci2=12.922 Ci3=18.728 | |
| R2 =0.476 | R2 =0.999 |
| 1 | GUO Fuyu, DING Changfeng, ZHOU Zhigao,et al.Assessment of the immobilization effectiveness of several amendments on a cadmium-contaminated soil using Eisenia fetida[J].Ecotoxicology and Environmental Safety,2020,189:109948. |
| 2 | 马凯悦,张浩,宋宁宁,等.氧化老化玉米秸秆生物炭吸附镉机理研究[J].农业环境科学学报,2022,41(6):1230-1240. |
| MA Kaiyue, ZHANG Hao, SONG Ningning,et al.Mechanism of cadmium adsorption by oxidative aging corn straw biochar[J].Journal of Agro-Environment Science,2022,41(6):1230-1240. | |
| 3 | 张欣,张丽.铝铁电极电絮凝法处理镀镉废水的研究[J].无机盐工业,2021,53(8):87-90. |
| ZHANG Xin, ZHANG Li.Study on the treatment of cadmiμm plating wastewater by electrocoagulation with Al-Fe electrode[J].Inorganic Chemicals Industry,2021,53(8):87-90. | |
| 4 | ZHANG Jian, ZHAN Siyan, ZHONG Lubin,et al.Adsorption of typical natural organic matter on microplastics in aqueous solution:Kinetics,isotherm,influence factors and mechanism[J].Journal of Hazardous Materials,2023,443:130130. |
| 5 | 林媛,弓晓峰,熊捷迁,等.外源DOM对湿地土壤吸附重金属Pb2+、Cd2+的影响[J].南昌大学学报(理科版),2021,45(1):71-78. |
| LIN Yuan, GONG Xiaofeng, XIONG Jieqian,et al.Effects of DOM on wetland soil adsorption of heavy metals Pb2+ and Cd2+ [J].Journal of Nanchang University(Natural Science),2021,45(1):71-78. | |
| 6 | 胡冬婉,马占玲,马骁,等.改性果胶-Fe3O4磁性微球制备及对Pb2+吸附性能[J].无机盐工业,2020,52(6):24-29. |
| HU Dongwan, MA Zhanling, MA Xiao,et al.Preparation of modified Fe3O4-pectin magnetic microspheres and its adsorption property for Pb2+ [J].Inorganic Chemicals Industry,2020,52(6):24-29. | |
| 7 | 谭雪云,李冰,李平,等.纳米羟基铁改性阳离子树脂制备及去除Cd(Ⅱ)[J].水处理技术,2019,45(6):56-60,65. |
| TAN Xueyun, LI Bing, LI Ping,et al.Preparation of cationic resin modified with nano-sized goethite and its application for Cd(Ⅱ) removal[J].Technology of Water Treatment,2019,45(6):56-60,65. | |
| 8 | WU C H, LIN Chengfang, MA H W,et al.Effect of fulvic acid on the sorption of Cu and Pb onto γ-Al2O3 [J].Water Research,2003,37(4):743-752. |
| 9 | 彭琪贵,施泽明,王新宇.针铁矿对水溶液中Cd2+、Pb2+的吸附影响[J].广州化工,2020,48(17):50-52,93. |
| PENG Qigui, SHI Zeming, WANG Xinyu.Effect of goethite on adsorption of Cd2+ and Pb2+ in aqueous solution[J].Guangzhou Chemical Industry,2020,48(17):50-52,93. | |
| 10 | 常洪铭,易筱筠,韦朝海.FeOOH对采矿废水中重金属的吸附[J].环境工程学报,2016,10(9):4956-4960. |
| CHANG Hongming, YI Xiaoyun, WEI Chaohai.Adsorption of heavy metal by FeOOH from mining wastewater[J].Chinese Journal of Environmental Engineering,2016,10(9):4956-4960. | |
| 11 | 罗畅.铁氧化物吸持腐殖酸对AsO4 3-、Hg2+次级吸附行为的影响研究[D].重庆:西南大学,2015. |
| LUO Chang.The secondary adsorption of AsO4 3- and Hg2+ on iron oxides complexed with humic acids[D].Chongqing:Southwest University,2015. | |
| 12 | LI Weiwei, ZHANG Fenfen, YE Qi,et al.Composition and copper binding properties of aquatic fulvic acids in eutrophic Taihu Lake,China[J].Chemosphere,2017,172:496-504. |
| 13 | FLEURY G, DEL NERO M, BARILLON R.Molecular fractionation of a soil fulvic acid (FA) and competitive sorption of trace metals (Cu,Zn,Cd,Pb) in hematite-solution systems:Effect of the FA-to-mineral ratio[J].RSC Advances,2017,7(68):43090-43103. |
| 14 | WU Shijiao, LU Jianwei, DING Zecong,et al.Cr(Ⅵ) removal by mesoporous FeOOH polymorphs:Performance and mechanism[J].RSC Advances,2016,6(85):82118-82130. |
| 15 | ORSETTI S,DE LAS MERCEDES QUIROGA M, ANDRADE E M.Binding of Pb(Ⅱ) in the system humic acid/goethite at acidic pH[J].Chemosphere,2006,65(11):2313-2321. |
| 16 | 管宇立.水铁矿及其与腐植酸共沉物对水溶液中镉的吸附作用研究[D].兰州:兰州大学,2018. |
| GUAN Yuli.Adsorption of cadmium from aqueous solution by ferrihydrite and their coprecipitate with humic acid[D].Lanzhou:Lanzhou University,2018. | |
| 17 | 张晶,郭学涛,葛建华,等.针铁矿-腐殖酸的复合物对泰乐菌素的吸附[J].环境工程学报,2016,10(3):1145-1151. |
| ZHANG Jing, GUO Xuetao, GE Jianhua,et al.Sorption of tylosin by goethite-humic acid complex[J].Chinese Journal of Environmental Engineering,2016,10(3):1145-1151. | |
| 18 | GUO Xuetao, YANG Chen, WU Yinai,et al.The influences of pH and ionic strength on the sorption of tylosin on goethite[J].Environmental Science and Pollution Research,2014,21(4):2572-2580. |
| 19 | 魏新涛.羟基氧化铁(FeOOH)及其复合物的制备与铀吸附性能研究[D].哈尔滨:哈尔滨工程大学,2018. |
| WEI Xintao.Study on the preparation of iron oxyhydroxide (FeOOH) and its complexes and the adsorption properties of uranium[D].Harbin:Harbin Engineering University,2018. | |
| 20 | 朱慧霞,张婷,韩琮,等.钾长石负载二氧化锰对Ni2+的吸附性能研究[J].无机盐工业,2020,52(1):44-48. |
| ZHU Huixia, ZHANG Ting, HAN Cong,et al.Study on adsorption performance of K-feldspar loaded MnO2 for Ni2+ [J].Inorganic Chemicals Industry,2020,52(1):44-48. | |
| 21 | 郭可心,田佳一,孙煜璨,等.磁性污泥基生物炭对Pb2+的吸附性能[J].环境工程学报,2022,16(5):1416-1428. |
| GUO Kexin, TIAN Jiayi, SUN Yucan,et al.Adsorption performance of magnetic sludge-derived biochar towards Pb2+ [J].Chinese Journal of Environmental Engineering,2022,16(5):1416-1428. | |
| 22 | 杨忠兰,曾希柏,孙本华,等.铁氧化物固定土壤重金属的研究进展[J].土壤通报,2021,52(3):728-735. |
| YANG Zhonglan, ZENG Xibai, SUN Benhua,et al.Research advances on the fixation of soil heavy metals by iron oxide[J].Chinese Journal of Soil Science,2021,52(3):728-735. | |
| 23 | GU Baohua, SCHMITT J, CHEN Zhihong,et al.Adsorption and desorption of natural organic matter on iron oxide:Mechanisms and models[J].Environmental Science & Technology,1994,28(1):38-46. |
| 24 | 翟羽佳,王建铎,王思达,等.腐殖酸与镉离子在水合二氧化锰表面的分配系数研究[J].环境科学学报,2015,35(10):3144-3150. |
| ZHAI Yujia, WANG Jianduo, WANG Sida,et al.Study on the distribution coefficients of humic acid and cadmium ions on the surface of hydrous manganese dioxides[J].Acta Scientiae Circumstantiae,2015,35(10):3144-3150. | |
| 25 | 谢发之,李海斌,李国莲,等.富里酸对针铁矿吸附Cr(Ⅵ)的影响机理[J].环境科学研究,2016,29(10):1506-1512. |
| XIE Fazhi, LI Haibin, LI Guolian,et al.Effects of fulvic acid on the adsorption of chromium(Ⅵ) to goethite[J].Research of Environmental Sciences,2016,29(10):1506-1512. | |
| 26 | FLOROIU R M, DAVIS A P, TORRENTS A.Cadmium adsorption on aluminum oxide in the presence of polyacrylic acid[J].Environmental Science & Technology,2001,35(2):348-353. |
| 27 | WANG Xiangxue, CHEN Zhongshan, TAN Xiaoli,et al.Effect of pH,humic acid and addition sequences on Eu(Ⅲ) sorption onto γ-Al2O3 study by batch and time resolved laser fluorescence spectroscopy[J].Chemical Engineering Journal,2016,287:313- 320. |
| 28 | 莫贞林,曾鸿鹄,林华,等.高锰酸钾改性桉木生物炭对Pb(Ⅱ)的吸附特性[J].环境科学,2021,42(11):5440-5449. |
| MO Zhenlin, ZENG Honghu, LIN Hua,et al.Adsorption characteristics of Pb(Ⅱ) on eucalyptus biochar modified by potassium permanganate[J].Environmental Science,2021,42(11):5440-5449. | |
| 29 | 曾丁才,吴宏海,林怡英,等.针铁矿/水界面反应性的实验研究[J].岩石矿物学杂志,2009,28(4):387-394. |
| ZENG Dingcai, WU Honghai, LIN Yiying,et al.An experimental study of goethite-water interface reaction[J].Acta Petrologica et Mineralogica,2009,28(4):387-394. | |
| 30 | 周代华,李学垣,徐凤琳,等.用质量作用模型描述重金属离子在土壤中的吸附特点[J].环境科学学报,1996,16(4):425-430. |
| ZHOU Daihua, LI Xueyuan, XU Fenglin,et al.Application of mass-action model for describing the absorption characteristics of heavy metals on soils[J].Acta Scientiae Circumstantiae,1996,16(4):425-430. |
| [1] | XU Mengyao, ZHANG Xin, HE Kunpeng, HE Jian, JIANG Wei. Preparation of yttrium oxide and zirconium phosphate adsorbents from zirconium-yttrium waste and evaluation of their performance [J]. Inorganic Chemicals Industry, 2024, 56(3): 116-124. |
| [2] | LI Qiaoyun, HUANG Xiuxing, WEI Wenye, CHEN Zhen. Study on adsorption of methylene blue by activated carbon with acid/alkali synergistically modified fly ash [J]. Inorganic Chemicals Industry, 2024, 56(3): 131-136. |
| [3] | LI Yang, LOU Feijian, SUI Xin, LI Keyan, LIU Fei, GUO Xinwen. Preparation of amine-functionalized fumed SiO2 materials and their performance for CO2 adsorption [J]. Inorganic Chemicals Industry, 2024, 56(2): 38-43. |
| [4] | ZHANG Li, ZHANG Dan, PAN Hongyan, DONG Yonggang, LI Wenfei, QIN Hong. Study on preparation of low ash activated carbon by phosphoric acid method [J]. Inorganic Chemicals Industry, 2024, 56(2): 95-103. |
| [5] | YAN Zhen, QIU Zhaofu, JIN Xibiao, WANG Yuan, LIU Chang, YANG Ji. Study on 4A molecular sieve loaded with Ce and γ-Fe2O3 for removal of Sb(Ⅲ) and Sb(Ⅴ) in water [J]. Inorganic Chemicals Industry, 2024, 56(1): 81-89. |
| [6] | ZHOU Shiqi, WANG Tao, JING Fangli, LUO Shizhong. Study on performance of magnesium nitrate-modified carbon molecular sieve for separation of nitrogen/methane [J]. Inorganic Chemicals Industry, 2023, 55(9): 75-80. |
| [7] | LI Chao, WANG Liping, DAI Yin, GAO Guimei, ZHANG Yunfeng, HONG Yu, XU Lijun, CUI Yongjie. Study on alkali fusion hydrothermal synthesis of 13X zeolite from high silicon tailings and its adsorption on lead,copper and zinc ions [J]. Inorganic Chemicals Industry, 2023, 55(9): 88-93. |
| [8] | WANG Yingnan, SHENG Linlin, HUANG Juan, HUANG Zhanbin. Study on adsorption performance of lead from water by coal-fired slag [J]. Inorganic Chemicals Industry, 2023, 55(8): 109-115. |
| [9] | HAN Hongjing, ZHANG Jingze, LAMAO Zhuoma, HAN Jizhe, WU Yongmin, TANG Weiping. Preparation of Al-Co co-doped lithium manganese oxide and its adsorption performance of lithium [J]. Inorganic Chemicals Industry, 2023, 55(7): 38-44. |
| [10] | FAN Fangfang, TONG Zhongkai, ZUO Weiyuan. Study on adsorption of tetracycline from wastewater by calcium modified peanut shell biochar [J]. Inorganic Chemicals Industry, 2023, 55(6): 109-115. |
| [11] | TIAN Yuling, CHENG Yang, HAN Rong, ZHOU Mei, WANG Chengjie, GE Qiangru. Effect of CaO on heavy metals stability and adsorption properties of sludge-derived biochar [J]. Inorganic Chemicals Industry, 2023, 55(6): 124-129. |
| [12] | FU Minglian, CEN Jianmei, CHEN Zhangxu. Study on preparation of magnetic SiO2/chitosan composite aerogel and its adsorption for Cu2+ [J]. Inorganic Chemicals Industry, 2023, 55(6): 70-77. |
| [13] | XU Chunhui, WANG Feng, LING Changjian, WANG Zirui, TANG Zhongfeng. Research progress of CO2 capture by metal oxides modified by molten salts [J]. Inorganic Chemicals Industry, 2023, 55(5): 1-7. |
| [14] | LIU Zihan, XI Guojun, LEI Guangping. Application of MOFs in adsorption refrigeration/heat pump [J]. Inorganic Chemicals Industry, 2023, 55(4): 20-26. |
| [15] | LU Jingjing,XIE Yan,LI Chen,MENG Mei,FENG Lunwei. Study on treatment of phosphorus-containing wastewater by lanthanum-loaded magnetized red mud [J]. Inorganic Chemicals Industry, 2023, 55(2): 99-105. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||