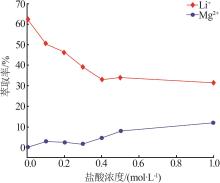
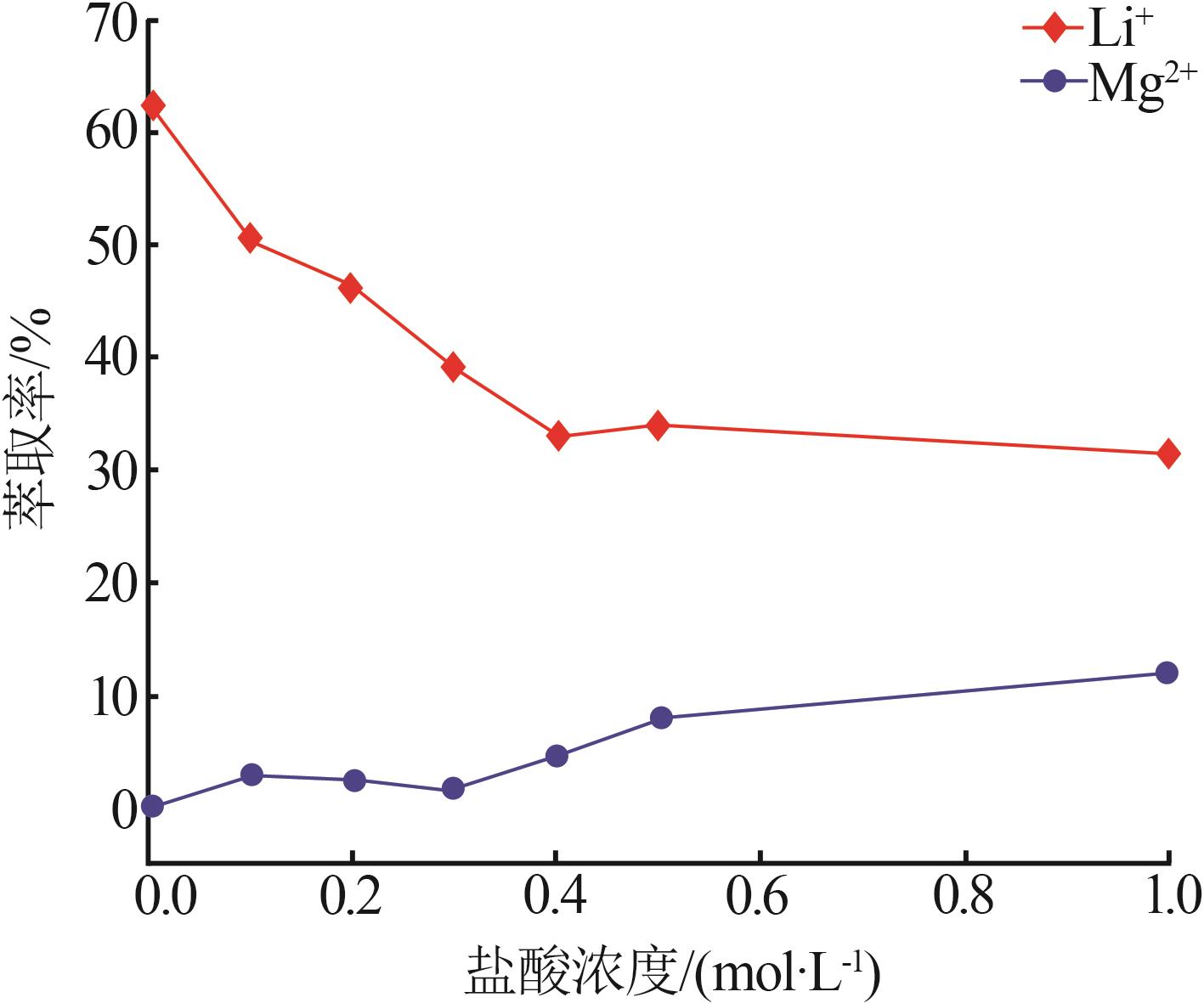
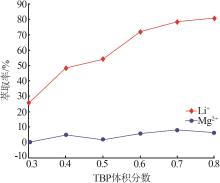
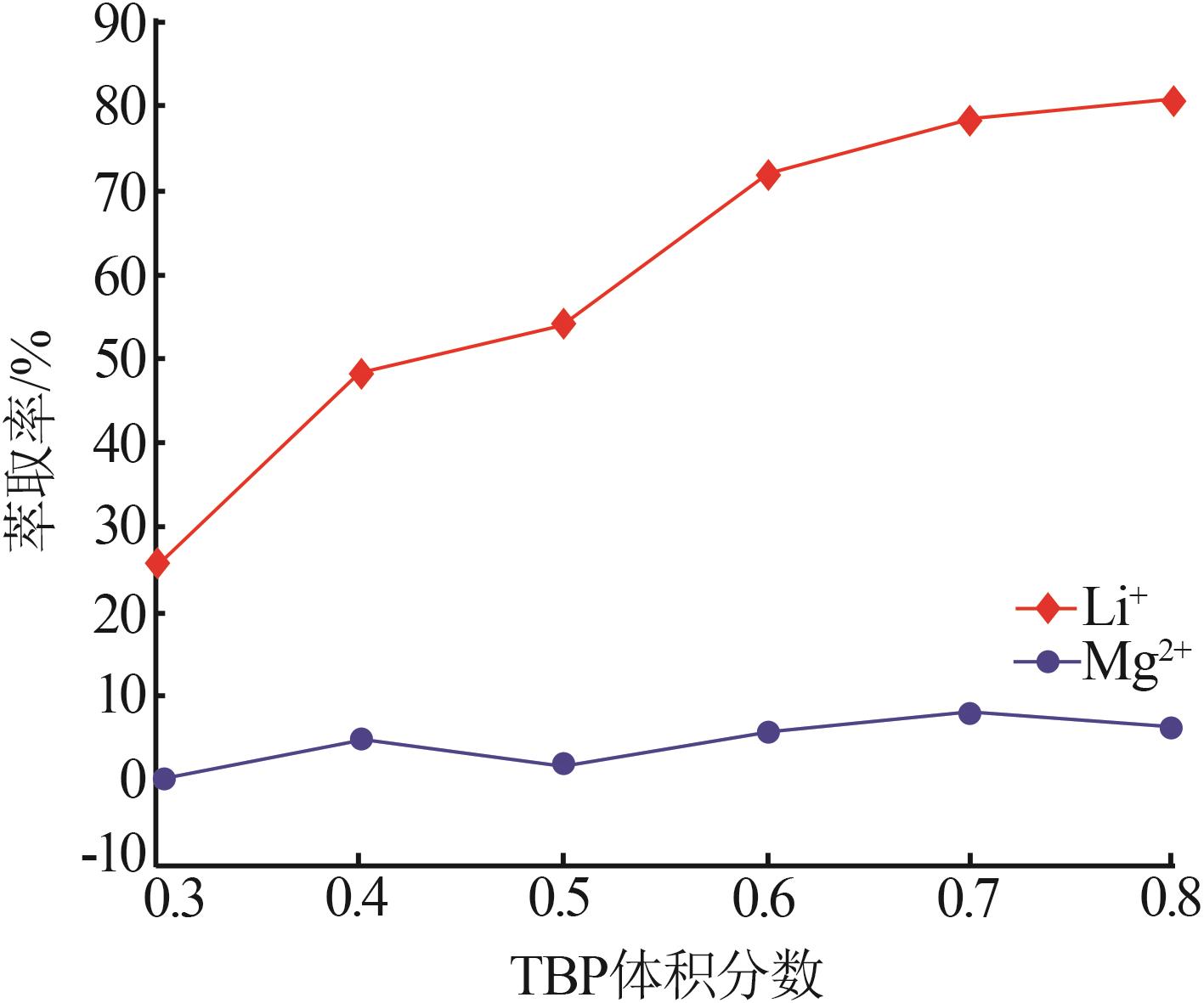
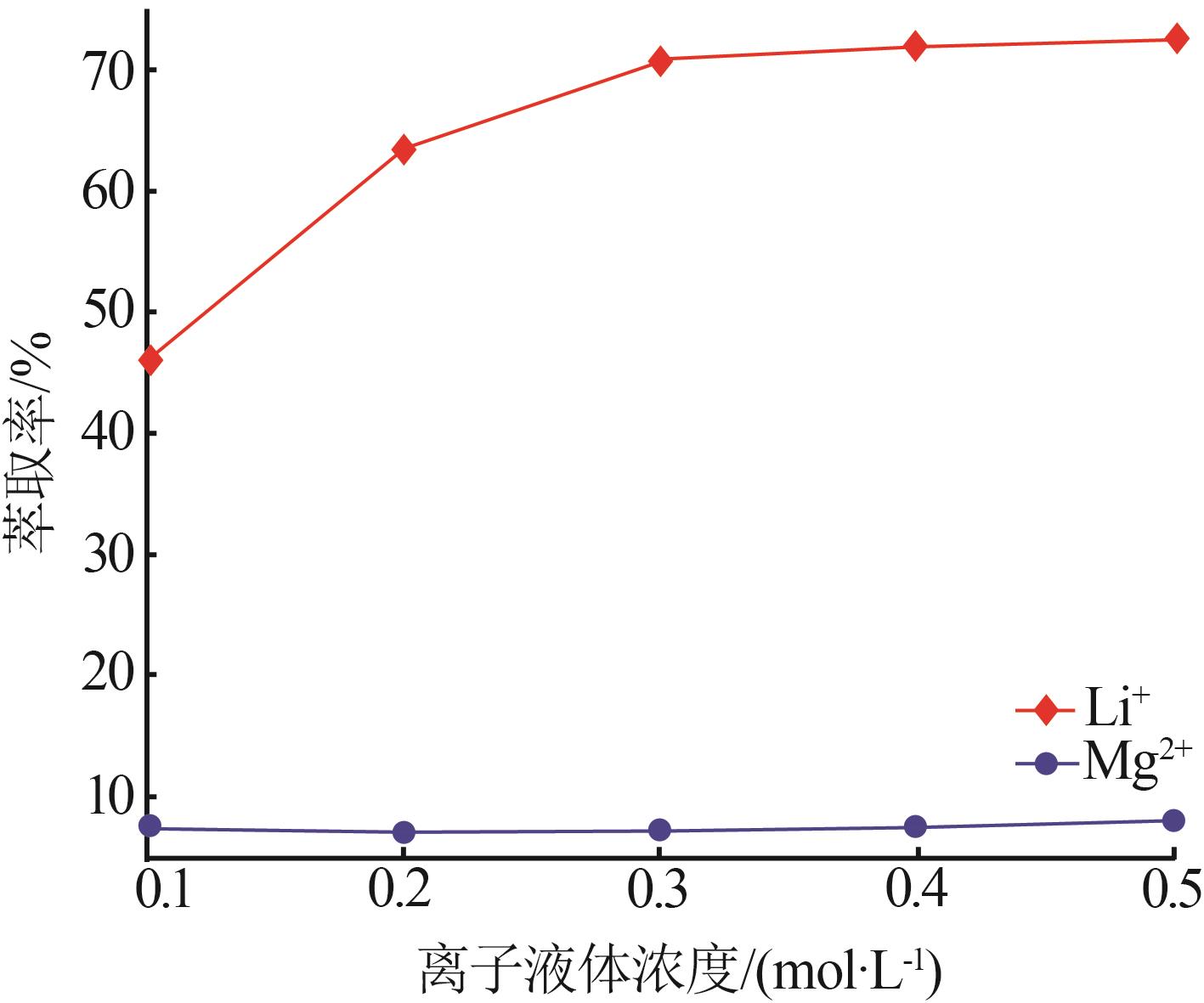
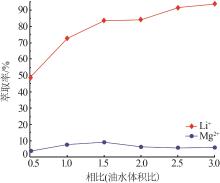
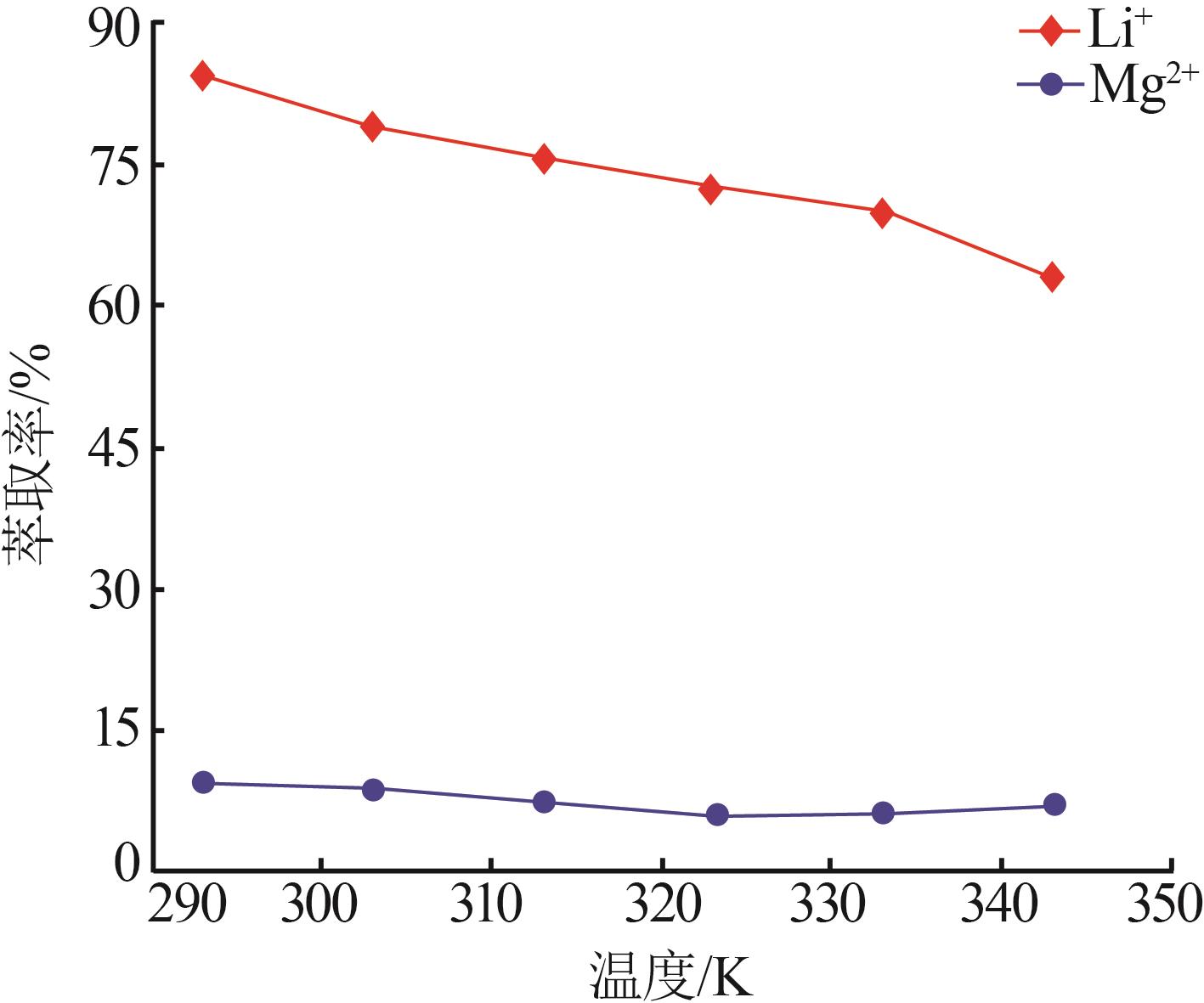
Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (7): 45-50.doi: 10.19964/j.issn.1006-4990.2022-0556
• Research & Development • Previous Articles Next Articles
Study on extraction of lithium from simulated old brine of salt lake by pyridine ionic liquid system
LU Nana1,2( ), QIN Yaru1,2, MA Shuqing1,2, WANG Qihui1,2, LIU Bing3, SHI Chenglong1,2(
), QIN Yaru1,2, MA Shuqing1,2, WANG Qihui1,2, LIU Bing3, SHI Chenglong1,2( )
)
- 1. School of Chemistry and Chemical Engineering,Qinghai Minzu University,Xining 810007,China
2. Key Laboratory of Resource Chemistry and Eco-environmental Protection in Tibetan Plateau,State Ethnic Affairs Commission,Xining 810007,China
3. Qinghai Institute of Salt Lakes,Chinese Academy of Sciences,Xining 810008,China
-
Received:2022-09-15Online:2023-07-10Published:2023-07-13 -
Contact:SHI Chenglong E-mail:shixiyuya@163.com;qhislscl@126.com
CLC Number:
Cite this article
LU Nana, QIN Yaru, MA Shuqing, WANG Qihui, LIU Bing, SHI Chenglong. Study on extraction of lithium from simulated old brine of salt lake by pyridine ionic liquid system[J]. Inorganic Chemicals Industry, 2023, 55(7): 45-50.
share this article
| 1 | 张婷,林森,于建国.磷酸铁锂正极材料的制备及性能强化研究进展[J].无机盐工业,2021,53(6):31-40. |
| ZHANG Ting, LIN Sen, YU Jianguo.Research progress in synthesis and performance enhancement of LiFePO4 cathode materials[J].Inorganic Chemicals Industry,2021,53(6):31-40. | |
| 2 | 黄杜斌,王培民,邬金龙.亲锂电解液在LiNi0 .6Mn 0.2Co0.2O2/Li二次锂金属电池中的电化学性能[J].应用化学,2022,39(2):298-306. |
| HUANG Dubin, WANG Peimin, WU Jinlong.Electrochemical performance of lithiophilic electrolyte in LiNi0.6Mn0.2Co0.2O2/Li rechargeable lithium metal battery[J].Chinese Journal of Applied Chemistry,2022,39(2):298-306. | |
| 3 | 陈倩倩,马典,林盼盼,等.磁性微晶玻璃连接锂铁氧体接头组织演变及磁性能模拟[J].机械工程学报,2022,58(4):34-40. |
| CHEN Qianqian, MA Dian, LIN Panpan,et al.Microstructure evolution of magnetic glass-ceramic brazed lithium ferrite joint and simulation of magnetic properties[J].Journal of Mechanical Engineering,2022,58(4):34-40. | |
| 4 | 陈士兵.二硅酸锂玻璃陶瓷在口腔磨损修复中的应用[J].粘接,2022,49(5):131-135. |
| CHEN Shibing.Application of lithium disilicate glass ceramic in oral wear repair[J].Adhesion,2022,49(5):131-135. | |
| 5 | 廖运文,赁敦敏,肖定全,等.钛酸铋钠钾锂无铅陶瓷的柠檬酸制备技术[J].无机盐工业,2006,38(2):19-21. |
| LIAO Yunwen, LIN Dunmin, XIAO Dingquan,et al.Bi0.5(Na1- x- y Li x K y )0.5TiO3 lead-free piezoelectric ceramics prepared by citrate-gel process[J].Inorganic Chemicals Industry,2006,38(2):19-21. | |
| 6 | 李林林,曹林娟,麦永雄,等.废旧锂离子电池有机酸湿法冶金回收技术研究进展[J].储能科学与技术,2020,9(6):1641-1650. |
| LI Linlin, CAO Linjuan, Yongxiong MAI,et al.Research progress of the organic acid of the hydrometallurgical recovery technology in spent Li ion batteries[J].Energy Storage Science and Technology,2020,9(6):1641-1650. | |
| 7 | 谢焕山,陈钢,陈宏镇,等.碳酸锂血药浓度与剂量比值的影响因素分析[J].中国临床药理学杂志,2020,36(9):1076-1078. |
| XIE Huanshan, CHEN Gang, CHEN Hongzhen,et al.Analysis of influence factors on serum concentration/dosage ratio of lithium carbonate[J].The Chinese Journal of Clinical Pharmacology,2020, 36(9):1076-1078. | |
| 8 | HE Maoyong, LUO Chongguang, YANG Hongjun,et al.Sources and a proposal for comprehensive exploitation of lithium brine deposits in the Qaidam basin on the northern Tibetan Plateau,China:Evidence from Li isotopes[J].Ore Geology Reviews,2020,117:103277. |
| 9 | 张业男,王韵珂,戴永年,等.共沉淀法合成高镍三元Ni0.8Co0.1Mn0.1(OH)2前驱体的可控制备[J].有色金属工程,2021,11(11):111-118. |
| ZHANG Yenan, WANG Yunke, DAI Yongnian,et al.Controlled fabrication of high nickel ternary Ni0.8Co0.1Mn0.1(OH)2 precursors by co-precipitation method[J].Nonferrous Metals Engineering,2021,11(11):111-118. | |
| 10 | 韩红静,吴勇民,曹永生,等.纳米片多面体锰基锂离子筛的制备及锂离子吸脱附特性[J].无机盐工业,2022,54(8):59-65. |
| HAN Hongjing, WU Yongmin, CAO Yongsheng,et al.Preparation of nano-flake polyhedral manganese based lithium ion sieve and its adsorption and desorption characteristic for Li[J].Inorganic Chemicals Industry,2022,54(8):59-65. | |
| 11 | 李燕,王敏,赵有璟,等.纳滤膜对高镁锂比盐湖卤水镁锂分离性能研究[J].化工学报,2021,72(6):3130-3139. |
| LI Yan, WANG Min, ZHAO Youjing,et al.Study on separation of magnesium and lithium from salt lake brine with high magnesium-to-lithium mass ratio by nanofiltration membrane[J].CIESC Journal,2021,72(6):3130-3139. | |
| 12 | 秦亚茹,石成龙,王兴权,等.离子液体体系卤水提锂的洗涤工艺研究[J].无机盐工业,2020,52(3):55-58,106. |
| QIN Yaru, SHI Chenglong, WANG Xingquan,et al.Study on scrubbing process of lithium extraction from brine in ionic liquid system[J].Inorganic Chemicals Industry,2020,52(3):55-58,106. | |
| 13 | ZHOU Zhiyong, LIU Haotian, FAN Jiahui,et al.Selective extraction of lithium ion from aqueous solution with sodium phosphomolybdate as a coextraction agent[J].ACS Sustainable Chemistry & Engineering,2019,7(9):8885-8892. |
| 14 | SU Hui, TAN Boren, ZHANG Jian,et al.Modelling of lithium extraction with TBP/P507-FeCl3 system from salt-lake brine[J].Separation and Purification Technology,2022,282:120110. |
| 15 | 谢铿,王海北.混盐型高锂卤水萃取分离锂[J].有色金属工程,2021,11(11):34-40. |
| XIE Keng, WANG Haibei.Extraction and separation of lithium from a salt lake brine with high lithium content[J].Nonferrous Metals Engineering,2021,11(11):34-40. | |
| 16 | 唐悦,叶国华,胡渝杰,等.离子液体在萃取分离中的应用现状与发展趋势[J].矿冶,2021,30(6):54-62. |
| TANG Yue, YE Guohua, HU Yujie,et al.Application status and development trend of ionic liquids in extraction and separation[J].Mining and Metallurgy,2021,30(6):54-62. | |
| 17 | BAI Ruibing, WANG Junfeng, CUI Li,et al.Efficient extraction of lithium ions from high Mg/Li ratio brine through the synergy of TBP and hydroxyl functional ionic liquids[J].Chinese Journal of Chemistry,2020,38(12):1743-1751. |
| 18 | LI Rujie, WANG Wanhang, WANG Yangyang,et al.Novel ionic liquid as co-extractant for selective extraction of lithium ions from salt lake brines with high Mg/Li ratio[J].Separation and Purification Technology,2021,277:119471. |
| 19 | ZHENG Hongshuai, DONG Tao, SHA Yifan,et al.Selective extraction of lithium from spent lithium batteries by functional ionic liquid[J].ACS Sustainable Chemistry & Engineering,2021, 9(20):7022-7029. |
| 20 | ZANTE G, TRÉBOUET D, BOLTOEVA M.Solvent extraction of lithium from simulated shale gas produced water with a bifunctional ionic liquid[J].Applied Geochemistry,2020,123:104783. |
| 21 | BAI Ruibing, WANG Junfeng, WANG Daoguang,et al.Selective separation of lithium from the high magnesium brine by the extraction system containing phosphate-based ionic liquids[J].Separation and Purification Technology,2021,274:119051. |
| 22 | WANG Xingquan, JING Yan, LIU Hong,et al.Extraction of lithium from salt lake brines by bis[(trifluoromethyl)sulfonyl]imide-based ionic liquids[J].Chemical Physics Letters,2018,707:8-12. |
| 23 | ZHOU Zhiyong, QIN Wei, LIANG Shengke,et al.Recovery of lithium using tributyl phosphate in methyl isobutyl ketone and FeCl3 [J].Industrial & Engineering Chemistry Research,2012,51:12926-12932. |
| 上接第 44 页) | |
| 24 | GU Donglei, SUN Wenjun, HAN Guofei,et al.Lithium ion sieve synthesized via an improved solid state method and adsorption performance for west taijinar salt lake brine[J].Chemical Engineering Journal,2018,350:474-483. |
| 25 | HAN H J, QU W, ZHANG Y L,et al.Enhanced performance of Li+ adsorption for H1.6Mn1.6O4 ion-sieves modified by Co doping and micro array morphology[J].Ceramics International,2021,47(15):21777-21784. |
| 26 | RYU T, HALDORAI Y, RENGARAJ A,et al.Recovery of lithium ions from seawater using a continuous flow adsorption column packed with granulated chitosan-lithium manganese oxide[J].Industrial & Engineering Chemistry Research,2016,55(26):7218-7225. |
| 27 | QIAN Fangren, GUO Min, QIAN Zhiqiang,et al.Highly lithium adsorption capacities of H1.6Mn1.6O4 ion-sieve by ordered array structure[J].ChemistrySelect,2019,4(34):10157-10163. |
| 28 | LAWAGON C P, NISOLA G M, CUEVAS R A I,et al.Development of high capacity Li+ adsorbents from H2TiO3/polymer nanofiber composites:Systematic polymer screening,characterization and evaluation[J].Journal of Industrial and Engineering Chemistry,2019,70:124-135. |
| 29 | WU Xianming, LI Runxiu, CHEN Shang,et al.Comparative study of Co,Cr and Al-doped LiMnO2 prepared by ion exchan- |
| ge[J].Bulletin of Materials Science,2008,31(2):109-113. | |
| 30 | CHEN Minmin, WU Ruyun, JU Shengui,et al.Improved performance of Al-doped LiMn2O4 ion-sieves for Li+ adsorption[J].Microporous and Mesoporous Materials,2018,261:29-34. |
| [1] | YANG Yousheng, YAO Zhihao, ZHAO Zhixing, FENG Xia, ZENG Ying, YU Xudong. Research progress of lithium-rich sulfate type salt lake brine evaporation experiment [J]. Inorganic Chemicals Industry, 2024, 56(4): 1-7. |
| [2] | WANG Haofang. Investigation of interfacial stability of Li1.3Al0.3Ti1.7(PO4)3 based all-solid-state lithium battery [J]. Inorganic Chemicals Industry, 2024, 56(4): 72-77. |
| [3] | ZHOU Haitao, WEN Chengqin, ZHENG Ling, SUN Jie. Research on boron nitride modified film for cathode interface of metallic lithium battery [J]. Inorganic Chemicals Industry, 2024, 56(4): 85-89. |
| [4] | LI Yaguang, HAN Dongzhan, QI Lijuan. Recent research on pretreatment of waste lithium-ion batteries and electrolyte recovery technology [J]. Inorganic Chemicals Industry, 2024, 56(2): 1-10. |
| [5] | PEI Hongchang, YUE Maowen, LIU Jianlu, LI Zhongfang, CHEN Xiaoyu. Research progress of comprehensive development and efficient utilization of seawater [J]. Inorganic Chemicals Industry, 2024, 56(2): 21-29. |
| [6] | YANG Shuiyan, YANG Mingxia, XIN Wanwan. Study on preparation and properties of high⁃purity lithium 2-trifluoromethyl-4,5-dicyanoimidazole [J]. Inorganic Chemicals Industry, 2024, 56(2): 74-79. |
| [7] | WANG Yanfei, YANG Chaofan, CAO Di, XU Shijie. Study on solid-liquid phase equilibrium of ternary system of Li+, Na+∥Cl--H2O at different temperatures [J]. Inorganic Chemicals Industry, 2024, 56(1): 33-39. |
| [8] | CHEN Haixia, YAN Hong, SUN Yunlong, MA Guoqiang. Research progress of lithium resource extraction technology [J]. Inorganic Chemicals Industry, 2024, 56(1): 9-22. |
| [9] | CHEN Tiandong, ZHAO Guangzhao, HAI Chunxi, DONG Shengde, HE Xin, XU Qi, FENG Hang, YUAN Shaoxiong, MA Luxiang, ZHOU Yuan. Research and industrialization progress on coating and doping modification of lithium-rich manganese-based materials [J]. Inorganic Chemicals Industry, 2023, 55(9): 1-8. |
| [10] | FU Yu, DENG Mi, HUANG Donggen, WAN Jinbao. Research progress of lithium extraction technology from salt lake brine [J]. Inorganic Chemicals Industry, 2023, 55(9): 9-16. |
| [11] | FENG Zhun. Improvement of high temperature stability of high nickel single crystal cathode materials by B/Al/Zr synergistic strategy [J]. Inorganic Chemicals Industry, 2023, 55(8): 59-64. |
| [12] | KANG Le, JING Maoxiang, LI Donghong, HU Xinyu, JIA Chunyan. Study on preparation and electrochemical performance of lithium aluminate nanorods modified solid electrolyte [J]. Inorganic Chemicals Industry, 2023, 55(8): 65-70. |
| [13] | WANG Hang, XU Chuan, YAN Xinxing, TU Mingjiang, CHEN Xin. Research progress of deep calcium removal process of battery-grade lithium carbonate [J]. Inorganic Chemicals Industry, 2023, 55(7): 18-24. |
| [14] | YU Hui, WANG Yubin, LIAO Zhejun, YANG Yunguang. Overview of recycling and utilization process of waste ternary lithium-ion power batteries [J]. Inorganic Chemicals Industry, 2023, 55(7): 32-37. |
| [15] | HAN Hongjing, ZHANG Jingze, LAMAO Zhuoma, HAN Jizhe, WU Yongmin, TANG Weiping. Preparation of Al-Co co-doped lithium manganese oxide and its adsorption performance of lithium [J]. Inorganic Chemicals Industry, 2023, 55(7): 38-44. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||