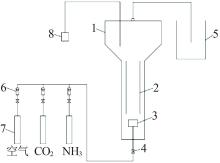
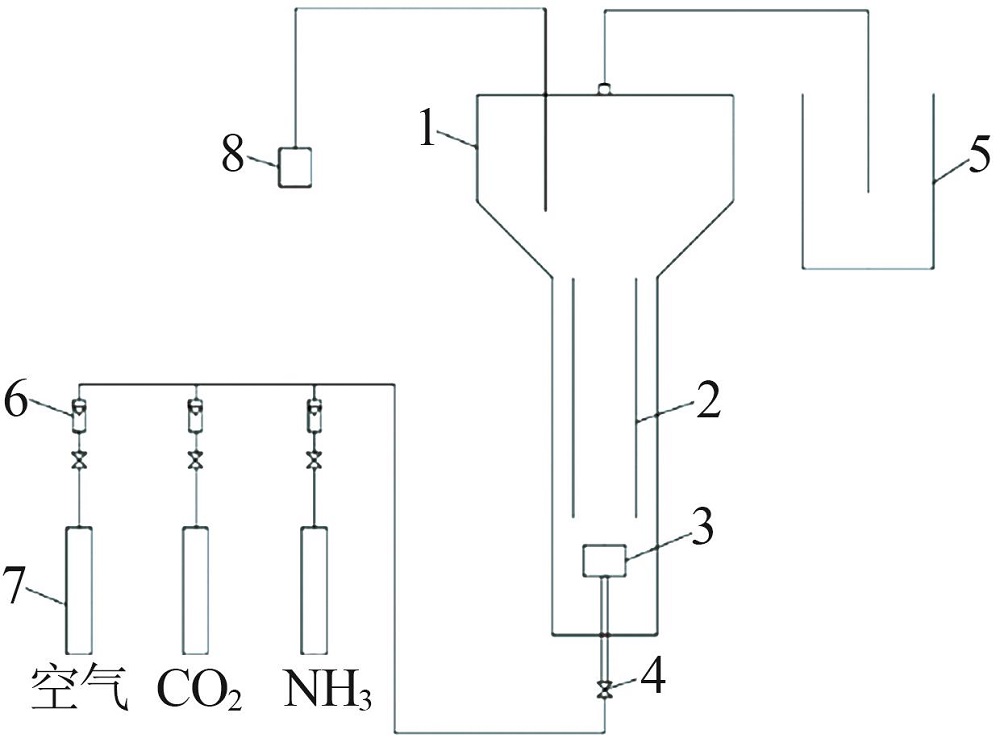
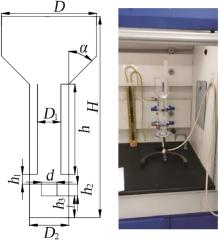
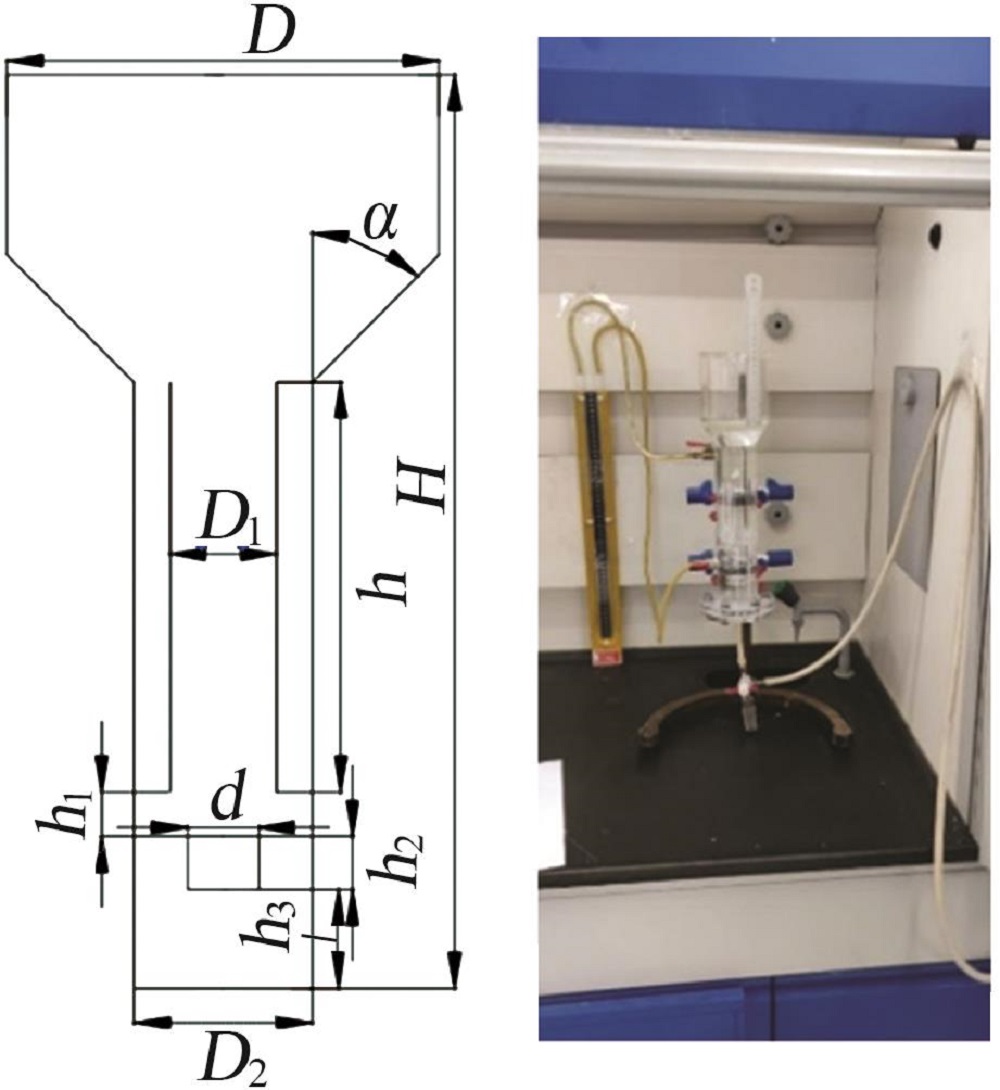
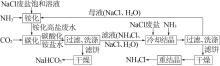
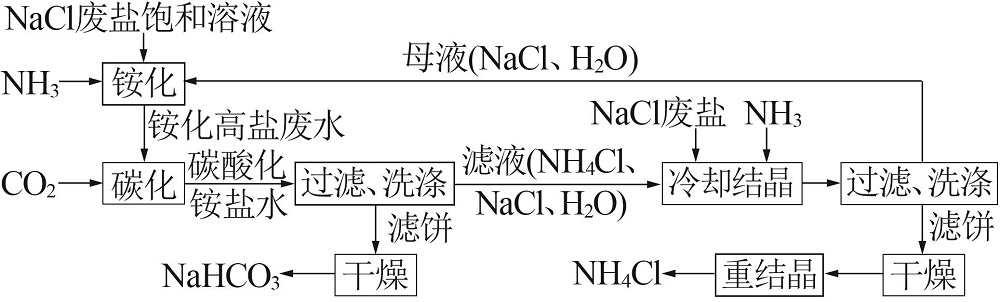
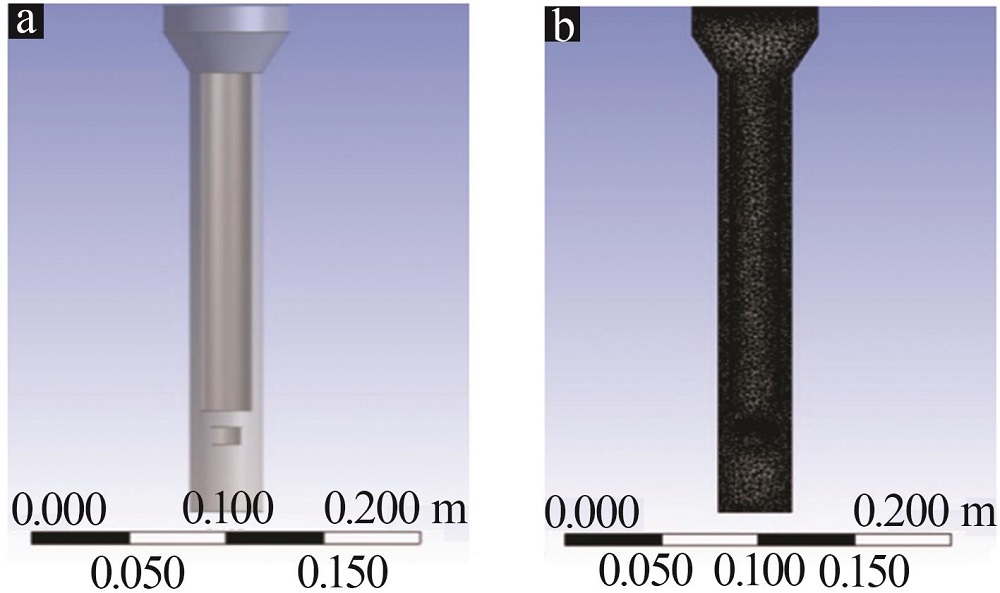
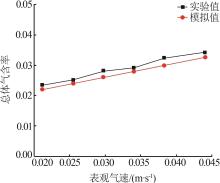
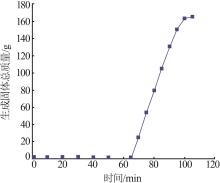
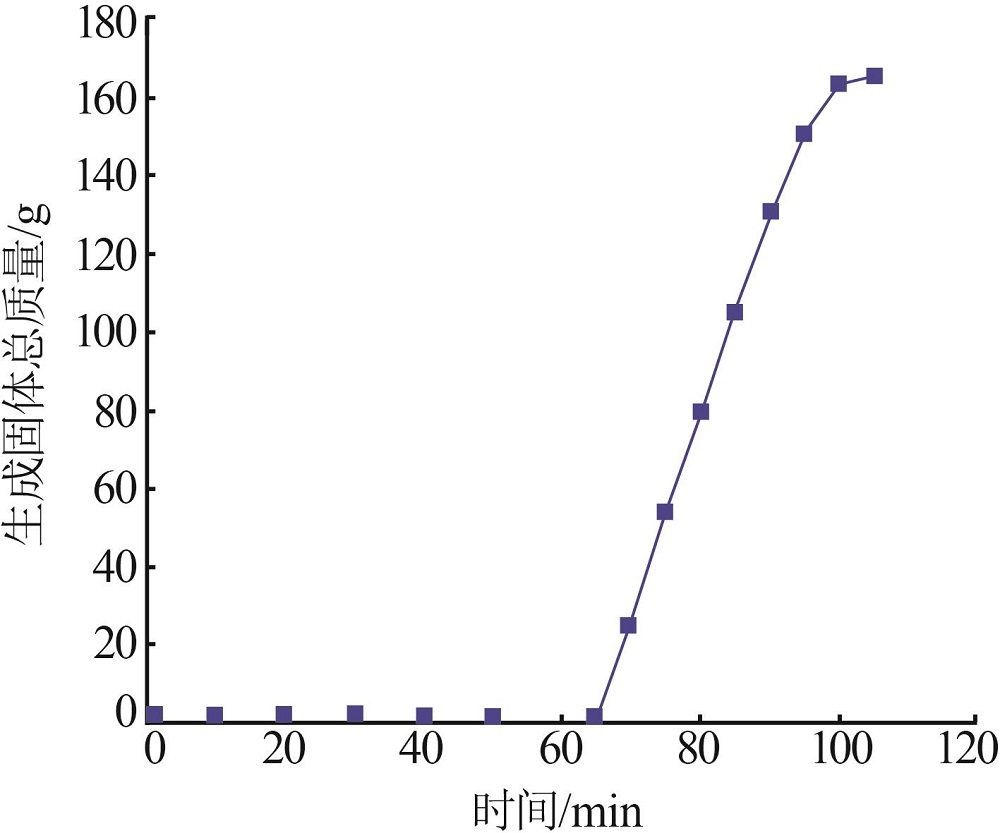
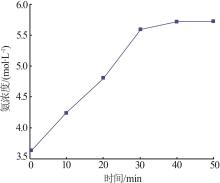
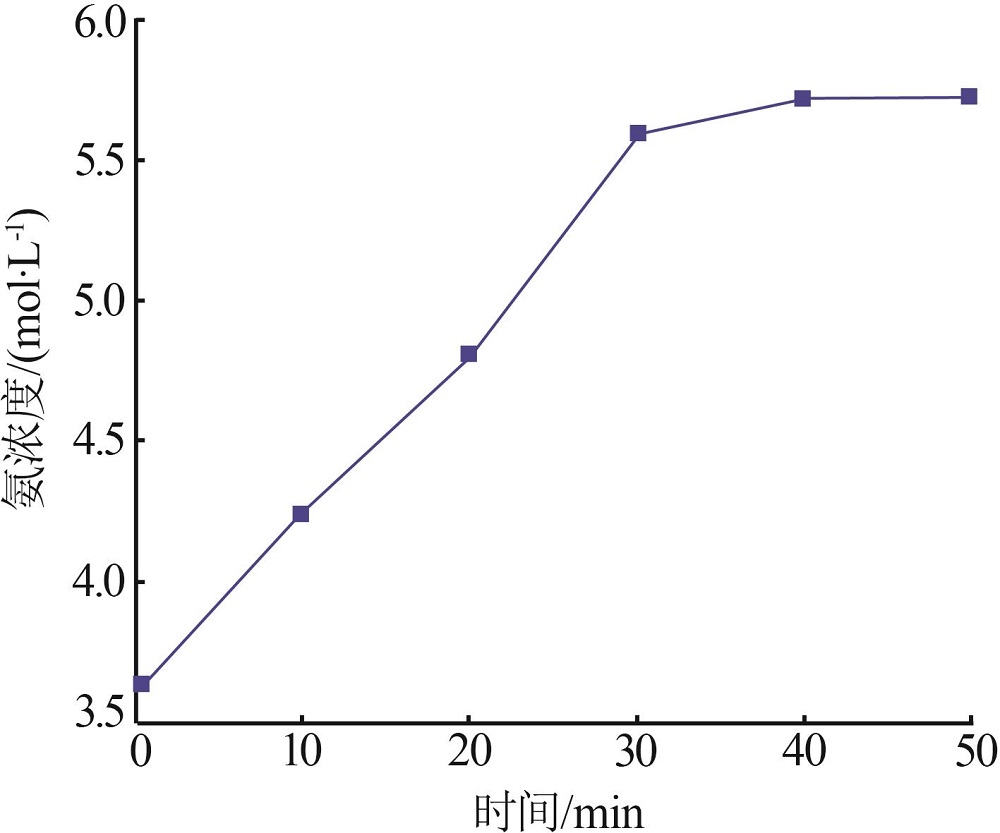
Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (12): 99-105.doi: 10.19964/j.issn.1006-4990.2022-0085
• Environment·Health·Safety • Previous Articles Next Articles
Study on intensification of gas-liquid reaction process for sodium chloride waste salt to ammonium alkali
SHEN Jiaqi1,2( ),YAN Shenghu1,2(
),YAN Shenghu1,2( ),ZHANG Yue2,LIU Jianwu1,2,SHEN Jiefa1,2
),ZHANG Yue2,LIU Jianwu1,2,SHEN Jiefa1,2
- 1. School of Petrochemical Engineering, School of Pharmacy, Changzhou University, Changzhou 213164, China
2. Continuous Flow Engineering Laboratory of National Petroleum and Chemical Industry
-
Received:2022-04-07Online:2022-12-10Published:2022-12-19 -
Contact:YAN Shenghu E-mail:3109957715@qq.com;ysh@cczu.edu.cn
CLC Number:
Cite this article
SHEN Jiaqi,YAN Shenghu,ZHANG Yue,LIU Jianwu,SHEN Jiefa. Study on intensification of gas-liquid reaction process for sodium chloride waste salt to ammonium alkali[J]. Inorganic Chemicals Industry, 2022, 54(12): 99-105.
share this article
| 1 | 苗淑兰, 陈侠, 周登凤, 等. 聚苯硫醚生产废水的资源化利用[J]. 化工进展, 2020, 39(10):4283-4289. |
| MIAO Shulan, CHEN Xia, ZHOU Dengfeng, et al. Resource utilization of polyphenylene sulfide production wastewater[J]. Chemical Industry and Engineering Progress, 2020, 39(10):4283-4289. | |
| 2 | 李绪宾, 刘会娥, 陈爽, 等. 工业废盐的流态化行为[J]. 化工进展, 2017, 36(1):81-90. |
| LI Xubin, LIU Hui′e, CHEN Shuang, et al. Fluidization behavior of industrial waste salt[J]. Chemical Industry and Engineering Progress, 2017, 36(1):81-90. | |
| 3 | 吕宏俊, 郭和民. 焚烧法处理有机废液的工艺选择[J]. 中国环保产业, 2005(12):36-38. |
| LV Hongjun, GUO Hemin. Process selection on organic waste liquors treated by incinerating method[J]. China Environmental Protection Industry, 2005(12):36-38. | |
| 4 | 丁志广, 郭键柄, 卢超. 化工废盐无害化处理的实验研究[J]. 无机盐工业, 2020, 52(2):58-61. |
| DING Zhiguang, GUO Jianbing, LU Chao. Experimental study on harmless disposal of chemical waste salts[J]. Inorganic Chemicals Industry, 2020, 52(2):58-61. | |
| 5 | 姜海超, 申银山, 陈晓飞, 等. 含氰工业废盐中杂质的高温氧化脱除实验研究[J]. 无机盐工业, 2020, 52(2):62-64. |
| JIANG Haichao, SHEN Yinshan, CHEN Xiaofei, et al. Experimental study on high temperature oxidation removal of impurities in cyanide-contained industrial waste salts[J]. Inorganic Chemicals Industry, 2020, 52(2):62-64. | |
| 6 | 郑虞琪, 刘志英, 李玲, 等. 热化学法降解农药废盐中的有机物[J]. 环境污染与防治, 2021, 43(7):822-828. |
| ZHENG Yuqi, LIU Zhiying, LI Ling, et al. Degradation of organics in pesticide waste salts by thermochemical method[J]. Environmental Pollution and Control, 2021, 43(7):822-828. | |
| 7 | 施云芬, 任惠敏, 于大禹. 气升式环流反应器处理废水的研究进展[J]. 东北电力大学学报, 2018, 38(4):76-84. |
| SHI Yunfen, REN Huimin, YU Dayu. Research progress on treatment of wastewater by airlift loop reactor[J]. Journal of Northeast Electric Power University, 2018, 38(4):76-84. | |
| 8 | LUO Huping, AL-DAHHAN M H. Local characteristics of hydrodynamics in draft tube airlift bioreactor[J]. Chemical Engineering Science, 2008, 63(11):3057-3068. |
| 9 | BEHIN J. Deinking in bubble column and airlift reactors:Influence of wastewater of Merox unit as pulping liquor[J]. Chemical Engineering Research and Design, 2012, 90(8):1045-1051. |
| 10 | ARUNKUMAR R, MUTHUKUMAR K. Phenomenological simulation model for the prediction of hydrodynamic parameters of an internal loop airlift reactor[J]. Industrial & Engineering Chemistry Research, 2010, 49: 4995-5000. |
| 11 | 张立英, 黄青山. 气升式环流反应器的理论研究进展[J]. 过程工程学报, 2011, 11(1):162-173. |
| ZHANG Liying, HUANG Qingshan. Research progress in the modeling theory of airlift loop reactor[J]. The Chinese Journal of Process Engineering, 2011, 11(1):162-173. | |
| 12 | 黄建新, 秦华明, 杨金水, 等. 外环流气升式反应器发酵生产聚β-羟基丁酸酯[J]. 西北大学学报:自然科学版, 2004(2):199-202. |
| HUANG Jianxin, QIN Huaming, YANG Jinshui, et al. Studies on producing poly-β-hydroxybutyrate in external loop airlift reactor[J]. Journal of Northwest University:Natural Science Edition, 2004(2):199-202. | |
| 13 | 闻建平, 潘磊, 白静. 多导流筒、低高径比气升环流反应器处理化肥厂含氨氮废水的中试研究[J]. 小氮肥, 2004(5):16-17. |
| WEN Jianping, PAN Lei, BAI Jing. Pilot study on the treatment of ammonia-containing nitrogen-containing wastewater in fertilizer plants with multiple diversion cylinders and low and high diameter specific airlift loop reactor[J]. Small Nitrogenous Fertilizer Plant, 2004(5):16-17. | |
| 14 | 乔萍, 闻建平. 气升式环流反应器合成2-氯-5-氯甲基吡啶[J]. 天津化工, 2002(5):4-5. |
| QIAO Ping, WEN Jianping. Synthesis of 2-chloro-5-(chloromet-hyl) pyridine by airlift loop reactor[J]. Tianjin Chemical Industry Journal, 2002(5):4-5. | |
| 15 | WAGH S M, KORANNE K V, SONOLIKAR R L. Operating and hydrodynamic characteristics of a reversed flow jet loop bioreactor with ejector[J]. Bioresource Technology, 2012, 110: 417-422. |
| 16 | GUO Xin, YAO Lishan, HUANG Qingshan. Aeration and mass transfer optimization in a rectangular airlift loop photobioreactor for the production of microalgae[J]. Bioresource Technology, 2015, 190: 189-195. |
| 17 | 郭秋丽, 赵德智, 刘永民, 等. 气升式环流反应器特性参数的研究进展[J]. 应用化工, 2017, 46(4):765-769. |
| GUO Qiuli, ZHAO Dezhi, LIU Yongmin, et al. Research progress on characteristic parameters of airlift loop reactor[J]. Applied Chemical Industry, 2017, 46(4):765-769. | |
| 18 | 李绍果, 薛天艳, 齐涛, 等. 分段进气多级环流反应器流动与传质特性研究[J]. 化学工程, 2013, 41(7):38-41. |
| LI Shaoguo, XUE Tianyan, QI Tao, et al. Flow and mass transfer characteristics of multi-sparger and multi-stage internal airlift loop reactor[J]. Chemical Engineering (China), 2013, 41(7):38-41. | |
| 19 | 张华海, 王铁峰. CFD-PBM耦合模型模拟气液鼓泡床的通用性研究[J]. 化工学报, 2019, 70(2):487-495. |
| ZHANG Huahai, WANG Tiefeng. Generality of CFD-PBM coupled model for simulations of gas-liquid bubble column[J]. CIESC Journal, 2019, 70(2):487-495. | |
| 20 | FELIX G, EMILIO G. Bioreactor scale-up and oxygen transfer rate in microbial processes:An overview[J]. Biotechnology Advances, 2009, 27(2):153-176. |
| [1] | YAO Jiankang, HU Shuozhen, NIU Dongfang, WU Jianping, ZHANG Xinsheng. Study on electrochemical treatment of sodium chloride organic waste salt in spice industry [J]. Inorganic Chemicals Industry, 2024, 56(3): 105-115. |
| [2] | YANG Fengling, QIAO Guoxin, YANG Pu, REN Lei, WANG Qiong, WU Haibin, CHENG Fangqin. Research progress and application of α-hemihydrate gypsum preparation from desulfurization gypsum [J]. Inorganic Chemicals Industry, 2024, 56(2): 11-20. |
| [3] | REN Teng, LI Shengdong, WANG Dexi, CHU Fuzhou, SHAO Lixin. Numerical simulation analysis of influence of jet position on mixing effect of carbonization reactor [J]. Inorganic Chemicals Industry, 2023, 55(7): 109-114. |
| [4] | LI Yu, ZHAO Wei, ZHANG Xinghua, WANG Rui, YAN Bingji, GUO Hongwei. Syudy on preparation of high-strength ceramsite from fluorite tailings and its properties [J]. Inorganic Chemicals Industry, 2023, 55(5): 100-108. |
| [5] | JIN Suna, LÜ Ruiliang. Research and application progress of wet flue gas desulfurization wastewater treatment technology [J]. Inorganic Chemicals Industry, 2023, 55(4): 27-37. |
| [6] | XU Wenzhen,LI Canhua,JI Hongfeng,LI Zimu,WU Zhaoyang,LI Minghui. Research progress of red mud in field of recycled metals and building materials [J]. Inorganic Chemicals Industry, 2023, 55(2): 10-18. |
| [7] | WANG Jinji, ZHAO Liang, ZHANG Menghui, XU Hanlu, DONG Hui. Numerical simulation of thermal characteristics in magnesium chloride pilot pyrolysis furnace [J]. Inorganic Chemicals Industry, 2023, 55(12): 140-145. |
| [8] | MIAO Linping,XU Miao,WANG Xinlong,YAN Zhengjuan,XU Dehua,YANG Jingxu. Study on preparation of calcium magnesium polyphosphate [J]. Inorganic Chemicals Industry, 2022, 54(9): 63-68. |
| [9] | GAO Run,YIN Jin,ZHANG Nan,GAO Ting,LIU Ruiyong. Current situation of waste salt generation and research progress on resource utilization in chemical industry [J]. Inorganic Chemicals Industry, 2022, 54(11): 25-31. |
| [10] | CHEN Xue,ZHANG Menghui,ZHAO Liang,DONG Hui,WANG Dexi. Study on pyrolysis process of Mg(NO3)2·nH2O [J]. Inorganic Chemicals Industry, 2021, 53(12): 91-94. |
| [11] | Shi Zhiping,Jiang Lan,Yang Hongliang,Zhang Jingzhou,Fu Gaofeng. Research status of recycling and resource utilization of aluminum dross [J]. Inorganic Chemicals Industry, 2020, 52(9): 21-25. |
| [12] | Chen Gang,Wen Yanshen,Peng Juan,Chen Changming,Gong Chuangzhou,Zhao Jie. Current situation and development direction of waste electronic chemical treatment and disposal standard in China [J]. Inorganic Chemicals Industry, 2020, 52(7): 18-21. |
| [13] | Ji Lichun. Present status of resource utilization of by-product green vitriol from titanium dioxide production by sulfuric acid method [J]. Inorganic Chemicals Industry, 2020, 52(5): 11-17. |
| [14] | Zhu Guihua,He Weize,Tang Haoting,Yi Shanzhen,Chen Yong. Simulation and improvement of flow field in side-entry wind salt mud drying room [J]. Inorganic Chemicals Industry, 2020, 52(12): 80-85. |
| [15] | Bian Xiaotong,Huang Yongming,Guo Rutao,Xu Donghua,Zhu Liangbing,Yang Ji,Qiu Zhaofu. Research progress on salt separation and resource utilization in high salinity wastewater [J]. Inorganic Chemicals Industry, 2019, 51(8): 7-12. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||