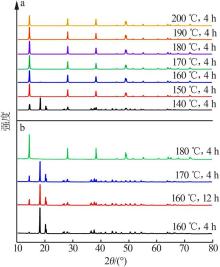
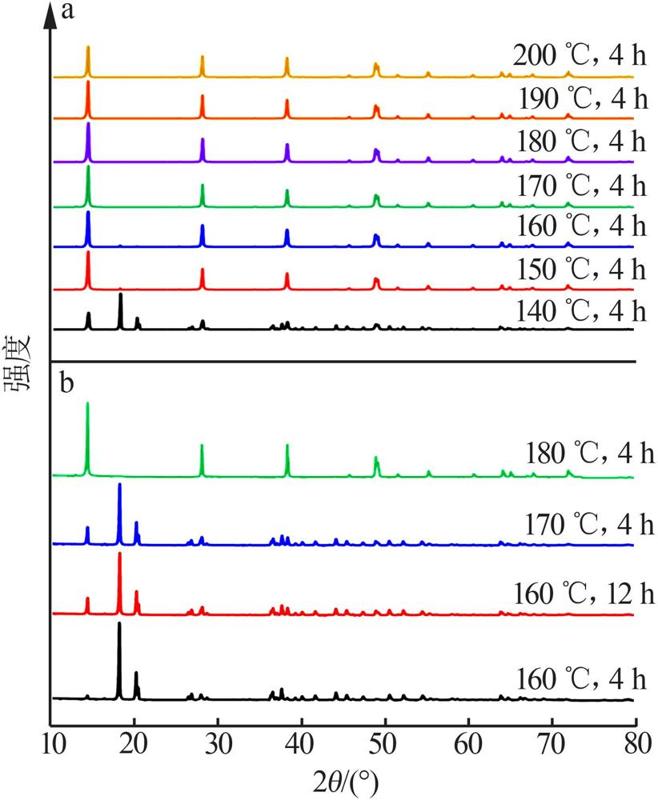
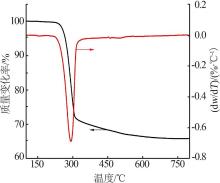
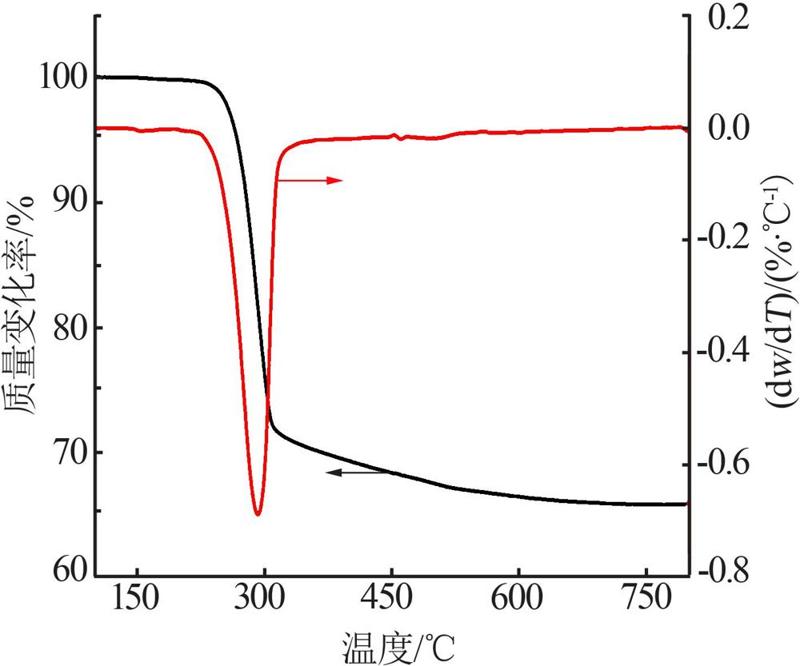
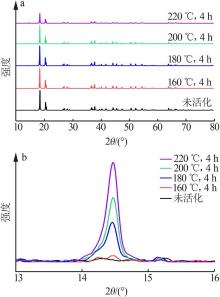
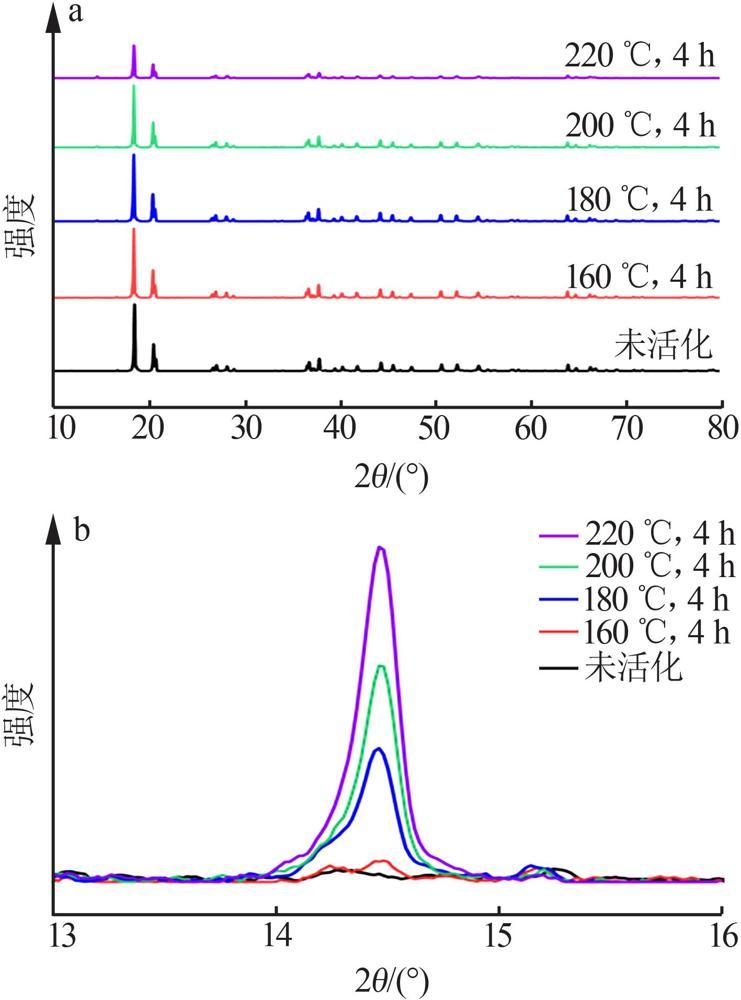
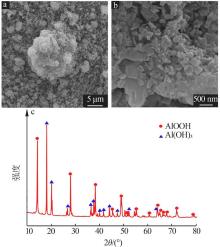
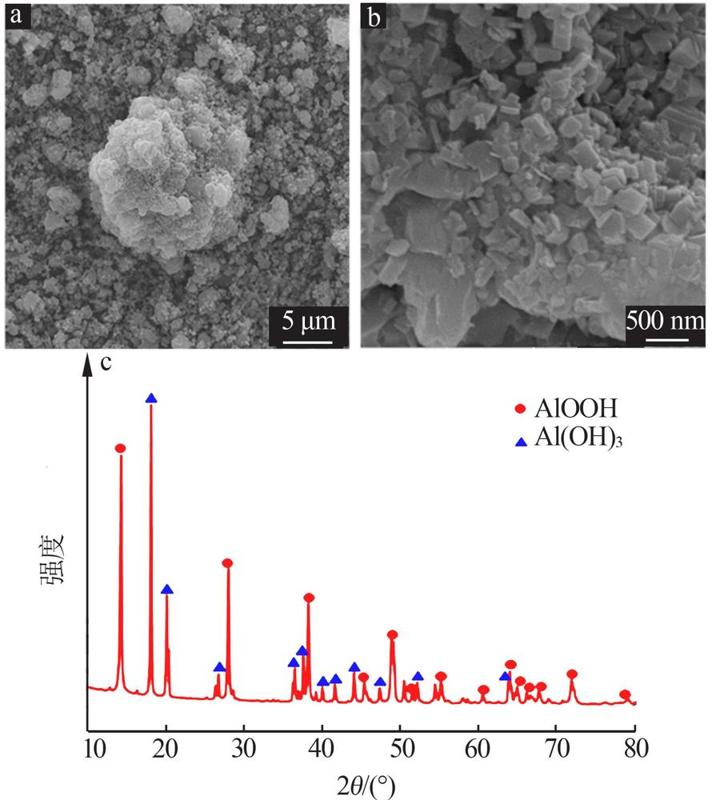
Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (9): 55-62.doi: 10.19964/j.issn.1006-4990.2021-0772
• Research & Development • Previous Articles Next Articles
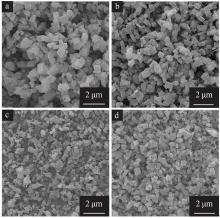
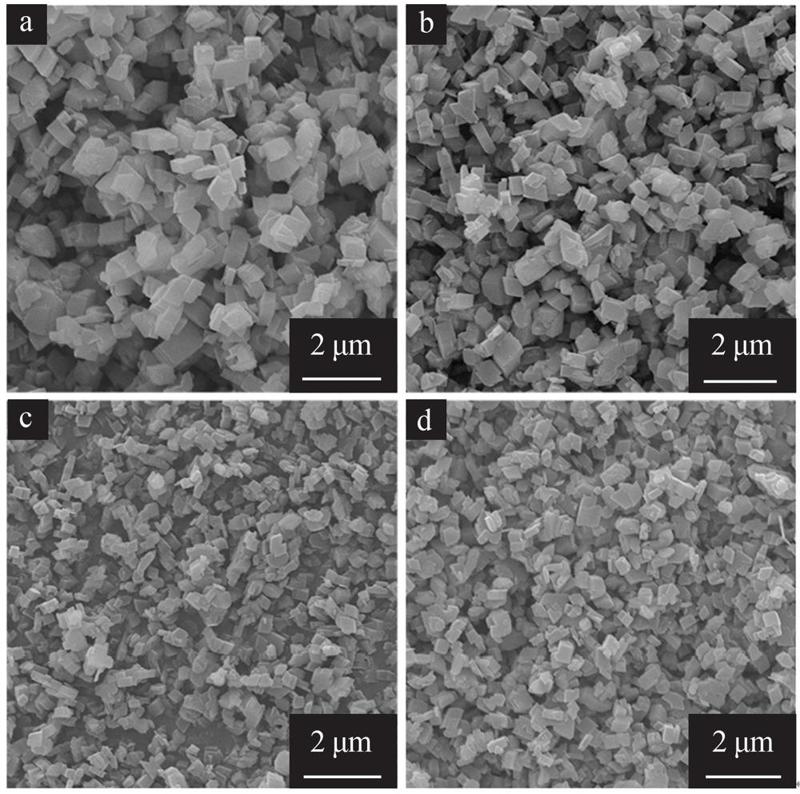
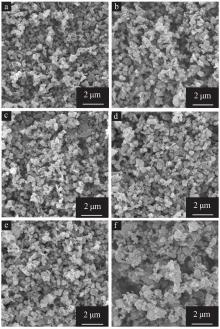
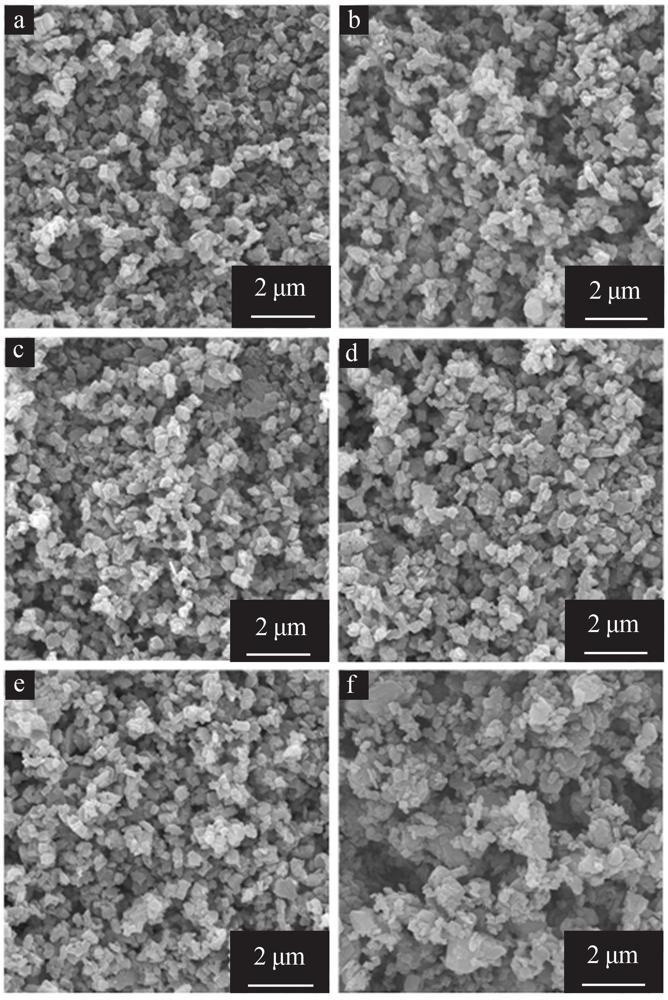
Effect of gibbsite activation on preparation of boehmite by hydrothermal method
YANG Yongyu1( ),TIAN Peng1,2(
),TIAN Peng1,2( ),ZHOU Ruohui1,XU Qianjin2,LIU Kunji2,NING Guiling1(
),ZHOU Ruohui1,XU Qianjin2,LIU Kunji2,NING Guiling1( )
)
- 1. Dalian University of Technology-Baohong Technology United Research Center for Advanced Lithium Battery Materials,School of Chemical Engineering,Dalian University of Technology,Dalian 116023,China
2. Jiangxi Baohtech Science Co.,Ltd.
-
Received:2021-12-23Online:2022-09-10Published:2022-09-22 -
Contact:TIAN Peng,NING Guiling E-mail:dllgyyy@mail.dlut.edu.cn;tianpeng@dlut.edu.cn;ninggl@dlut.edu.cn
CLC Number:
Cite this article
YANG Yongyu,TIAN Peng,ZHOU Ruohui,XU Qianjin,LIU Kunji,NING Guiling. Effect of gibbsite activation on preparation of boehmite by hydrothermal method[J]. Inorganic Chemicals Industry, 2022, 54(9): 55-62.
share this article
| [1] | PARDO P, SERRANO F J, VALLCORBA O, et al.Enhanced lateral to basal surface ratio in boehmite nanoparticles achieved by hydrothermal aging[J].Crystal Growth & Design,2015,15(7):3532-3538. |
| [2] |
KANBARGI N, LESSER A J.Improving adhesion between aramid fibers and natural rubber through morphological and synthetic modification of the fibers[J].Journal of Applied Polymer Science,2018,135(24).Doi:10.1002/app.45520.
doi: 10.1002/app.45520. |
| [3] | OH C J, YI Y K, KIM S J, et al.Production of micro⁃crystalline boehmite from hydrothermal processing of Bayer plant alumina tri⁃hydrate[J].Powder Technology,2013,235:556-562. |
| [4] | WANG Song, ZHAO Guofeng, LIU Ye, et al.Microfibrous⁃structured Pd/AlOOH/Al-fiber with hydroxyl⁃enriched surfaces for the catalytic semihydrogenation of acetylene[J].Industrial & Engineer⁃ ing Chemistry Research,2019,58(36):16431-16441. |
| [5] | 杨永钰,高婷婷,田朋,等.无机超细粉体改性锂离子电池隔膜的研究进展[J].无机盐工业,2021,53(6):49-58. |
| YANG Yongyu, GAO Tingting, TIAN Peng, et al.Research progress of lithium⁃ion battery separator modified with inorganic ultrafine powder[J].Inorganic Chemicals Industry,2021,53(6):49-58 | |
| [6] | LI Yaqian, YU Le, HU Weiren, et al.Thermotolerant separators for safe lithium⁃ion batteries under extreme conditions[J].Journal of Materials Chemistry A,2020,8(39):20294-20317. |
| [7] | 李长刚,赵萍,王维勋,等.微乳/水热法制备勃姆石[J].齐鲁工业大学学报,2017,31(5):13-16. |
| LI Changgang, ZHAO Ping, WANG Weixun, et al.Preparation of boehmite by microemulsion/hydrothermal method[J].Journal of Qilu University of Technology,2017,31(5):13-16. | |
| [8] | DUBEY S P, DWIVEDI A D, SILLANPÄÄ M, et al.Adsorption of As(V) by boehmite and alumina of different morphologies prepared under hydrothermal conditions[J].Chemosphere,2017,169:99-106. |
| [9] | JIANG Ru, LIU Haitao, CHENG Haifeng.Characterization for thermal evolution of boehmite precursor and preparation of alumina through a sol-gel process[J].Rare Metal Materials and Engineering,2018,47:188-192. |
| [10] | 李帅帅,高志华,刘琰,等.催化剂中AlOOH比例对浆态床一步法合成二甲醚的影响[J].天然气化工:C1化学与化工,2018,43(2):29-33. |
| LI Shuaishuai, GAO Zhihua, LIU Yan, et al.Effect of AlOOH ratio in catalyst on one⁃step synthesis of dimethyl ether in slurry bed[J].Natural Gas Chemical Industry,2018,43(2):29-33. | |
| [11] | CHEN Xianfu, ZHANG Wei, LIN Yuqing, et al.Preparation of high⁃flux γ-alumina nanofiltration membranes by using a modified sol-gel method[J].Microporous and Mesoporous Materials,2015,214:195-203. |
| [12] | TCHOMGUI-KAMGA E, AUDEBRAND N, DARCHEN A.Effect of co⁃existing ions during the preparation of alumina by electrolysis with aluminum soluble electrodes:Structure and defluoridation activity of electro⁃synthesized adsorbents[J].Journal of Hazardous Materials,2013,254/255:125-133. |
| [13] | STAROWICZ M, STAROWICZ P, STYPUŁA B.Alumina⁃based nanoparticles obtained by anodic dissolution of Al in electrolytes with alcohol solvents[J].Journal of Solid State Electrochemistry,2014,18(11):3065-3071. |
| [14] |
OHTA Y, HAYAKAWA T, INOMATA T, et al.Novel nano boehmite prepared by solvothermal reaction of aluminum hydroxide gel in monoethanolamine[J].Journal of Nanoparticle Resear⁃ ch,2017,19(7).Doi:10.1007/s11051-017-3918-3.
doi: 10.1007/s11051-017-3918-3. |
| [15] | 关昕,王晶,史忠祥.水热法制备薄水铝石粉体及其脱水动力学分析[J].大连交通大学学报,2018,39(4):77-82. |
| GUAN Xin, WANG Jing, SHI Zhongxiang.Preparation and kinetics of dehydration of boehmite by hydrothermal treatment[J].Journal of Dalian Jiaotong University,2018,39(4):77-82. | |
| [16] | OHTA Y, HAYAKAWA T, INOMATA T, et al.A blue photoluminescent nano boehmite prepared by a solvothermal reaction of aluminum hydroxide gel in monoethanolamine at low temperature[J].Chemistry Letters,2017,46(1):32-34. |
| [17] | 杨铎,史忠祥,王晶,等.多相氢氧化铝向薄水铝石相转变过程的微观结构研究[J].功能材料,2021,52(4):4147-4152. |
| YANG Duo, SHI Zhongxiang, WANG Jing, et al.Study on microstructure of multiphase aluminum hydroxide transition to boehmite phase[J].Journal of Functional Materials,2021,52(4):4147-4152. | |
| [18] | 左少卿,杜晓辉,熊晓云,等.拟薄水铝石制备方法的研究进展[J].应用化工,2017,46(9):1818-1821. |
| ZUO Shaoqing, DU Xiaohui, XIONG Xiaoyun, et al.Research progress in preparation of pseudo⁃boehmite[J].Applied Chemical Industry,2017,46(9):1818-1821. | |
| [19] | SHEN S,NG W, CHIA L, et al.Morphology controllable synthesis of nanostructured boehmite and γ-alumina by facile dry gel conversion[J].Crystal Growth & Design,2012,12(10):4987-4994. |
| [20] | PANASYUK G P, KOZEROZHETS I V, SEMENOV E A, et al.Mechanism of phase transformations of γ-Al2O3 and Al(OH)3 into boehmite(AlOOH) during hydrothermal treatment[J].Inorganic Materials,2019,55(9):929-933. |
| [21] | ZHANG Xin, CUI Wenwen, HU Jianzhi, et al.Transformation of gibbsite to boehmite in caustic aqueous solution at hydrothermal conditions[J].Crystal Growth & Design,2019,19(10):5557-5567. |
| [22] | MEHTA S K, KALSOTRA A.Kinetics and hydrothermal transformation of gibbsite[J].Journal of Thermal Analysis,1991,37(2):267-275. |
| [23] | JIAO Wenqian, WU Xuezhong, XUE Teng, et al.Morphological controlled growth of nanosized boehmite with enhanced aspect ratios in an organic additive⁃free cationic⁃anionic double hydrolysis method[J].Crystal Growth & Design,2016,16(9):5166-5173. |
| [24] | KOZEROZHETS I V, PANASYUK G P, SEMENOV E A, et al.How acid medium affects the hydrothermal synthesis of boehmi⁃te[J].Russian Journal of Inorganic Chemistry,2020,65(10):1529-1534. |
| [25] | LI Zheng, LIU Guihua, LI Xiaobin, et al.Effects of cation on the morphology of boehmite precipitated from alkaline solutions by adding gibbsite as seed[J].Crystal Growth & Design,2019,19(3):1778-1785. |
| [26] | HUANG Wenqiang, LIU Guihua, QI Tiangui, et al.Effects of pH and ions on the morphological evolution of boehmite prepared by hydrothermal treatment of ultrafine Bayer gibbsite[J].CrystEngComm,2020,22(42):6983-6992. |
| [27] | TSUCHIDA T.Hydrothermal synthesis of submicrometer crystals of boehmite[J].Journal of the European Ceramic Society,2000,20(11):1759-1764. |
| [28] | ZHANG Xin, CUI Wenwen, PAGE K L, et al.Size and morphology controlled synthesis of boehmite nanoplates and crystal growth mechanisms[J].Crystal Growth & Design,2018,18(6):3596-3606. |
| [29] | 周俊文,王建立,宋为聪.低钠薄水铝石耐热阻燃剂的开发及应用[J].轻金属,2014(5):16-19. |
| ZHOU Junwen, WANG Jianli, SONG Weicong.Development and application of boehmite with low sodium for heat⁃resistant flame retardant[J].Light Metals,2014(5):16-19. | |
| [30] | 高振昕,贺中央,郑小平,等.拜尔法三水铝石受热相变的形貌特征[J].硅酸盐学报,2008,36(S1):117-123. |
| GAO Zhenxin, HE Zhongyang, ZHENG Xiaoping, et al.Phase transformation and morphology of Bayer⁃gibbsite during heating[J].Journal of the Chinese Ceramic Society,2008,36(S1):117-123. | |
| [31] | 陈博,陈小明,蒋学鑫.水和水蒸气辅助粗晶三水铝石转化为勃姆石的机制研究[J].岩石矿物学杂志,2021,40(6):1197-1202. |
| CHEN Bo, CHEN Xiaoming, JIANG Xuexin.The transformation mechanism of the macrogranular gibbsite to boehmite assisted by water and water vapor[J].Acta Petrologica et Mineralogica,2021,40(6):1197-1202. | |
| [32] | 杨会宾,孙俊民,胡剑,等.三水铝石水热处理过程中的相变及其影响[J].轻金属,2017(2):13-16,28. |
| YANG Huibin, SUN Junmin, HU Jian, et al.Phase transition and influences of gibbsite in the hydrothermal process[J].Light Metals,2017(2):13-16,28. | |
| [33] | 王迎,俞小花,冯攀,等.拜耳法制备高纯氧化铝过程中水热深度脱钠[J].过程工程学报,2018,18(1):148-153. |
| WANG Ying, YU Xiaohua, FENG Pan, et al.Deeply removing sodium by hydrothermal method in the process of preparing high pure alumina from bayer liquid[J].The Chinese Journal of Process Engineering,2018,18(1):148-153. | |
| [34] | 许珂敬,杨新春,田贵山,等.采用引入晶种的水热合成法制备α-Al2O3纳米粉[J].硅酸盐学报,2001,29(6):576-579. |
| XU Kejing, YANG Xinchun, TIAN Guishan, et al.Preparation of nano⁃size α-Al2O3 powders by seeding hydrothermal synthesizing method[J].Journal of the Chinese Ceramic Society,2001,29(6):576-579. |
| [1] | DI Lu, WANG Weiguo, CHEN Juexian, WU Chuanshu. Study on preparation of transition metal-supported Silicalite-1 zeolite catalyst and its catalytic performance for furfural hydrogenation [J]. Inorganic Chemicals Industry, 2024, 56(4): 125-132. |
| [2] | LI Chao, WANG Liping, DAI Yin, GAO Guimei, ZHANG Yunfeng, HONG Yu, XU Lijun, CUI Yongjie. Study on alkali fusion hydrothermal synthesis of 13X zeolite from high silicon tailings and its adsorption on lead,copper and zinc ions [J]. Inorganic Chemicals Industry, 2023, 55(9): 88-93. |
| [3] | DING Hongyu, ZHONG Jianchu, WANG Hongzhi, ZHANG Shuang. Hydrothermal synthesis of talc by crystal seed induction method [J]. Inorganic Chemicals Industry, 2023, 55(5): 59-65. |
| [4] | CHEN Zhangxu, FU Minglian, ZHU Danchen, ZHENG Bingyun. Preparation of carbon/graphite carbon nitride composites and their methylene blue removal performance [J]. Inorganic Chemicals Industry, 2023, 55(3): 134-139. |
| [5] | ZHANG Xing,XU Jie,WANG Zibing,HOU Peng,HE Long,LIU Huan. Effect of feedstock particle size on kinetics of limestone thermal decomposition reaction [J]. Inorganic Chemicals Industry, 2023, 55(2): 79-84. |
| [6] | YANG Fengling,ZHAI Min,REN Lei,ZHANG Yuanyuan,CHENG Fangqin,DONG Hongyu. Influencing factors of crystallization products in wet desulfurization of carbide slag [J]. Inorganic Chemicals Industry, 2023, 55(2): 92-98. |
| [7] | DONG Xiongwei, HAN Fenglan, HUA Wei, LI Maohui, AN Changcong, HUANG Yucai. Synthesis and characterization of silico-manganese slag zeolite [J]. Inorganic Chemicals Industry, 2023, 55(12): 128-132. |
| [8] | JIANG Demin, LI Shunmei, Li Qingqing, Chen Yuxin, LIU Daijun. Hydrothermal synthesis of basic magnesium sulfate whiskers from magnesium hydroxide and magnesium sulfate heptahydrate [J]. Inorganic Chemicals Industry, 2023, 55(12): 74-81. |
| [9] | PENG Qiugui, YANG Fan, LÜ Renliang, XIANG Lan. Effect of pre-reduction on deferrization in hydrothermal hydrolysis process of Mg/Al/Fe-bearing TiOSO4 solution [J]. Inorganic Chemicals Industry, 2023, 55(12): 95-101. |
| [10] | TENG Jiayang, FENG Qingge, ZHANG Xuan, QIN Fanghong, FENG Jinghang, HU Jiawen, CHEN Chaohong. Study on preparation of pseudo-boehmite from aluminum dross resource treatment [J]. Inorganic Chemicals Industry, 2023, 55(11): 130-138. |
| [11] | TIAN Peng, ZHOU Ruohui, XU Qianjin, LIU Kunji, PANG Hongchang, NING Guiling. Synthesis and dehydration dynamics of boehmite microcrystalline with different particle sizes [J]. Inorganic Chemicals Industry, 2023, 55(11): 27-36. |
| [12] | LU Yang,WANG Jing,HE Lijie,WANG Fudong,ZHANG Jiaming. Research progress on preparation of magnesium aluminate spinel powders and it in field of luminescent material [J]. Inorganic Chemicals Industry, 2022, 54(9): 39-46. |
| [13] | JIANG Tiantian,XU Xuetang,WANG Fan. Research on growth and supercapacitance of NiCo based electrode materials regulated by halogen ions [J]. Inorganic Chemicals Industry, 2022, 54(8): 66-73. |
| [14] | LI Fan,YANG Yuzhe,TIAN Peng,LI Wei,NING Guiling. Preparation of high purity pseudo-boehmite by hydrolysis of aluminium sec-butoxide [J]. Inorganic Chemicals Industry, 2022, 54(7): 78-84. |
| [15] | XU Qingying,YANG Dingyi,LÜ Wei,LI Xiang,QIAN Yunfeng,DU Baocong. Effect of grinding time on properties of phosphogypsum based cementitious materials [J]. Inorganic Chemicals Industry, 2022, 54(5): 101-108. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||