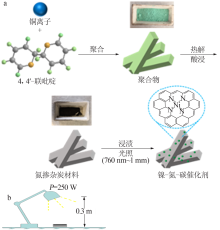
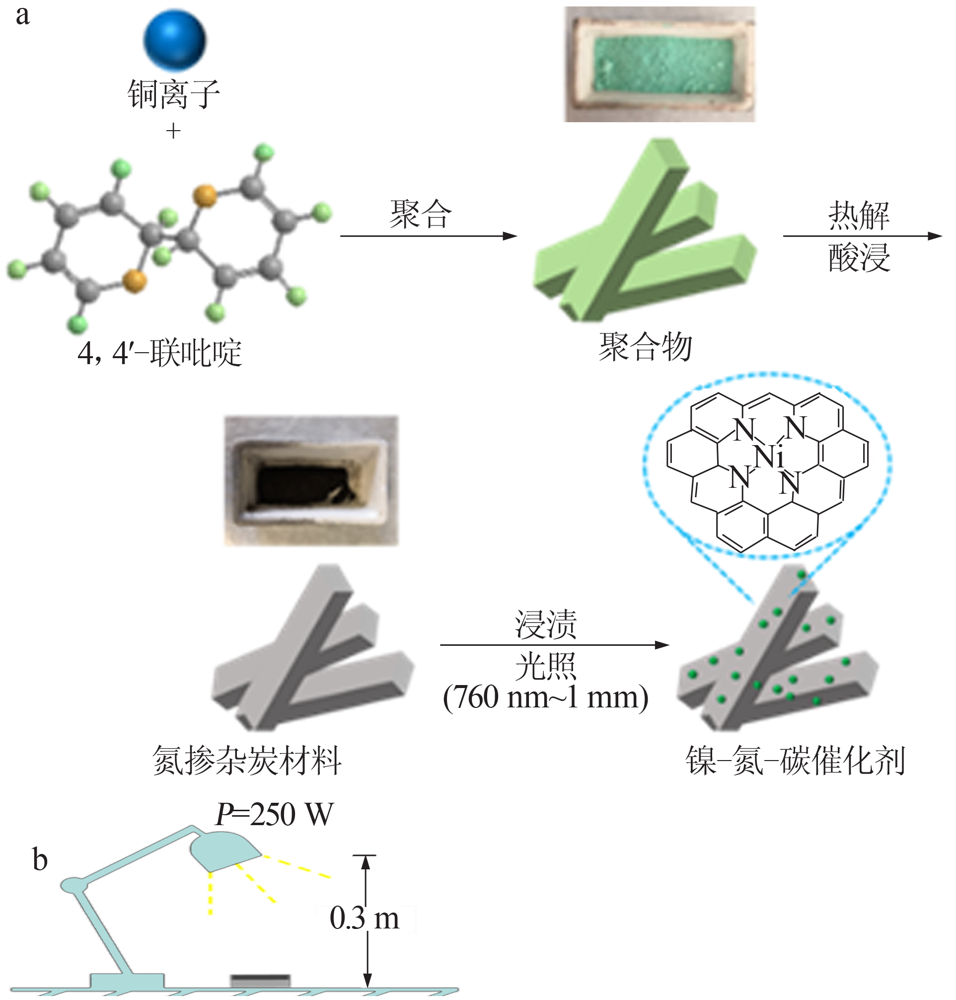
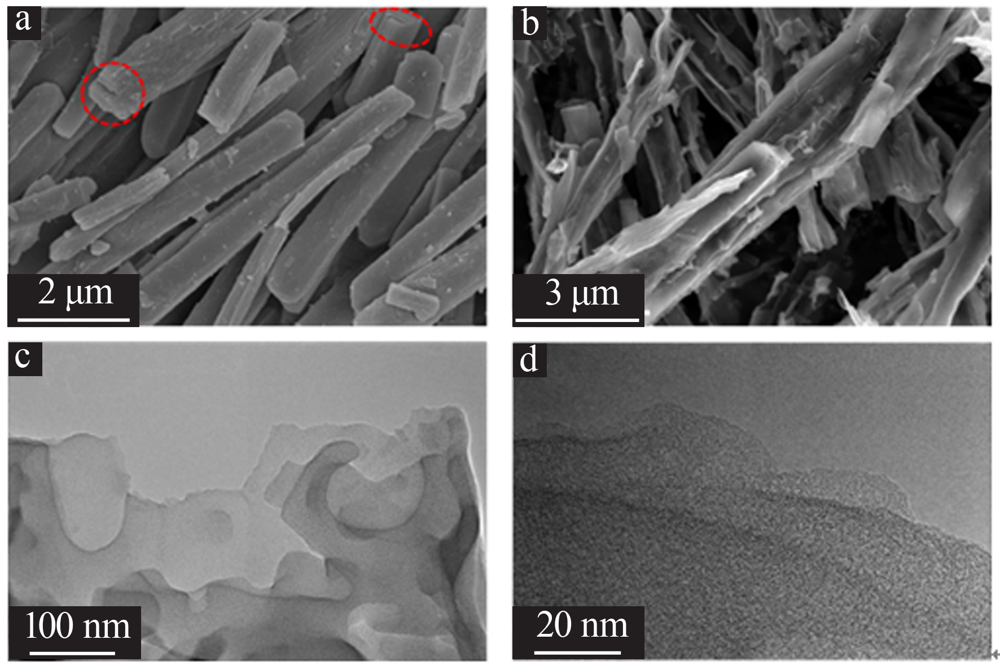
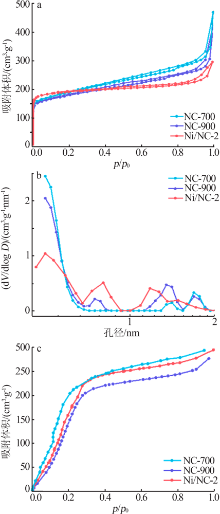
| [1] |
Verma S, Lu S, Kenis P J A. Co-electrolysis of CO2 and glycerol as a pathway to carbon chemicals with improved technoeconomics due to low electricity consumption[J]. Nature Energy, 2019,4(6):466-474.
doi: 10.1038/s41560-019-0374-6
|
| [2] |
Koshy D M, Chen S, Lee D U, et al. Understanding the origin of hi-ghly selective CO2 electroreduction to CO on Ni,N-doped carbon ca-talysts[J]. Angewandte Chemie International Edition, 2020,59(10):4043-4050.
doi: 10.1002/anie.v59.10
|
| [3] |
Han L, Sun Y Y, Li S, et al. In-plane carbon lattice-defect regulating electrochemical oxygen reduction to hydrogen peroxide production over nitrogen-doped graphene[J]. ACS Catalysis, 2019,9(2):1283-1288.
doi: 10.1021/acscatal.8b03734
|
| [4] |
Huan T N, Ranjbar N, Rousse G, et al. Electrochemical reduction of CO2 catalyzed by Fe-N-C materials:A structure-selectivity study[J]. ACS Catalysis, 2017,7(3):1520-1525.
doi: 10.1021/acscatal.6b03353
|
| [5] |
Varela A S, Ju W, Bagger A, et al. Electrochemical reduction of CO2 on metal-nitrogen-doped carbon catalysts[J]. ACS Catalysis, 2019,9(8):7270-7284.
doi: 10.1021/acscatal.9b01405
|
| [6] |
Ye C, Yu X, Li W, et al. Engineering of bifunctional nickel phosphi-de@Ni-N-C catalysts for selective electroreduction of CO2-H2O to syngas[J]. Acta Physico-Chimica Sinica, 2020.Doi: 10.3866/PKU.WHXB202004054.
|
| [7] |
Möller T, Ju W, Bagger A, et al. Efficient CO2 to CO electrolysis on solid Ni-N-C catalysts at industrial current densities[J]. Energy & Environmental Science, 2019,12(2):640-647.
|
| [8] |
Zheng T, Jiang K, Ta N, et al. Large-scale and highly selective CO2 electrocatalytic reduction on nickel single-atom catalyst[J]. Joule, 2019,3(1):265-278.
doi: 10.1016/j.joule.2018.10.015
|
| [9] |
Gong Y N, Jiao L, Qian Y, et al. Regulating the coordination environ-ment of MOF-templated single-atom nickel electrocatalysts for boo-sting CO2 reduction[J]. Angewandte Chemie International Edition, 2020,132(7):2727-2731.
|
| [10] |
Zhang L, Han L, Liu H, et al. Potential-cycling synjournal of single platinum atoms for efficient hydrogen evolution in neutral media[J]. Angewandte Chemie International Edition, 2017,56(44):13694-13698.
doi: 10.1002/anie.201706921
|
| [11] |
Zhao C, Wang Y, Li Z, et al. Solid-diffusion synjournal of single-atom catalysts directly from bulk metal for efficient CO2 reduction[J]. Joule, 2019,3(2):584-594.
doi: 10.1016/j.joule.2018.11.008
|
| [12] |
Alam M Z, De Leon I, Boyd R W. Large optical nonlinearity of in-dium tin oxide in its epsilon-near-zero region[J]. Science, 2016,352(6287):795-797.
doi: 10.1126/science.aae0330
|
| [13] |
Wei H, Wu H, Huang K, et al. Ultralow-temperature photochemical synjournal of atomically dispersed Pt catalysts for the hydrogen evo-lution reaction[J]. Chemical Science, 2019,10(9):2830-2836.
doi: 10.1039/C8SC04986F
|
| [14] |
Yang M, Allard L F, Flytzani-Stephanopoulos M. Atomically dis-persed Au-(OH)x species bound on titania catalyze the low-tempe-rature water-gas shift reaction[J]. Journal of the American Chemi-cal Society, 2013,135(10):3768-3771.
|
| [15] |
Li T F, Liu J J, Song Y, et al. Photochemical solid-phase synjournal of platinum single atoms on nitrogen-doped carbon with high load-ing as bifunctional catalysts for hydrogen evolution and oxygen re-duction reactions[J]. ACS Catalysis, 2018,8(9):8450-8458.
doi: 10.1021/acscatal.8b02288
|
| [16] |
Kishi N, Fukaya A, Sugita R, et al. Synjournal of graphenes on Ni fo-ils by chemical vapor deposition of alcohol with IR-lamp heating[J]. Materials Letters, 2012,79:21-24.
doi: 10.1016/j.matlet.2012.03.084
|
| [17] |
Zhang T, Han X, Yang H, et al. Atomically dispersed nickel(I) on an alloy-encapsulated nitrogen-doped carbon nanotube array for high-performance electrochemical CO2 reduction reaction[J]. An-gewandte Chemie International Edition, 2020,59(29):12055-12061.
|
| [18] |
Wen C F, Mao F X, Liu Y W, et al. Nitrogen-stabilized low-valent Ni motifs for efficient CO2 electrocatalysis[J]. ACS Catalysis, 2020,10(2):1086-1093.
doi: 10.1021/acscatal.9b02978
|
| [19] |
Zhou W, Shen H, Wang Q, et al. N-doped peanut-shaped carbon nanotubes for efficient CO2 electrocatalytic reduction[J]. Carbon, 2019,152:241-246.
doi: 10.1016/j.carbon.2019.05.078
|
 ),Dong Lingyu,Li Wencui,Hao Guangping(
),Dong Lingyu,Li Wencui,Hao Guangping( )
)