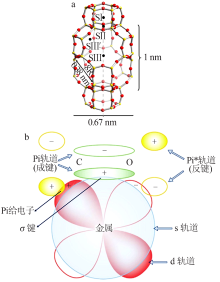
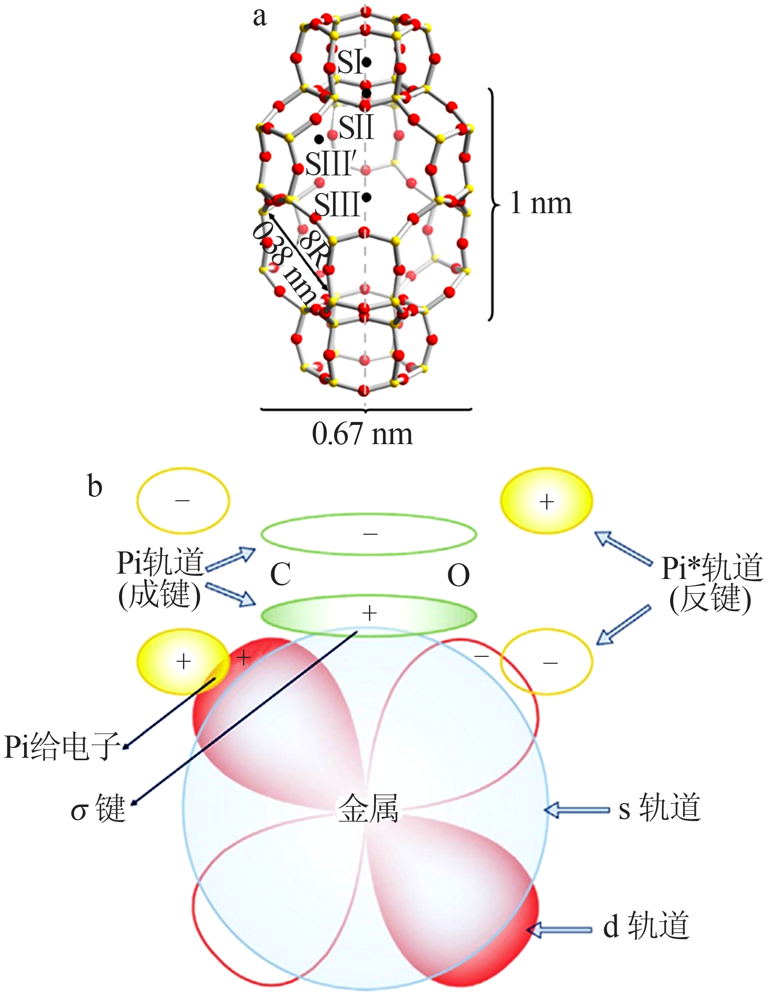
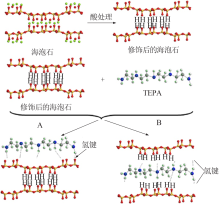
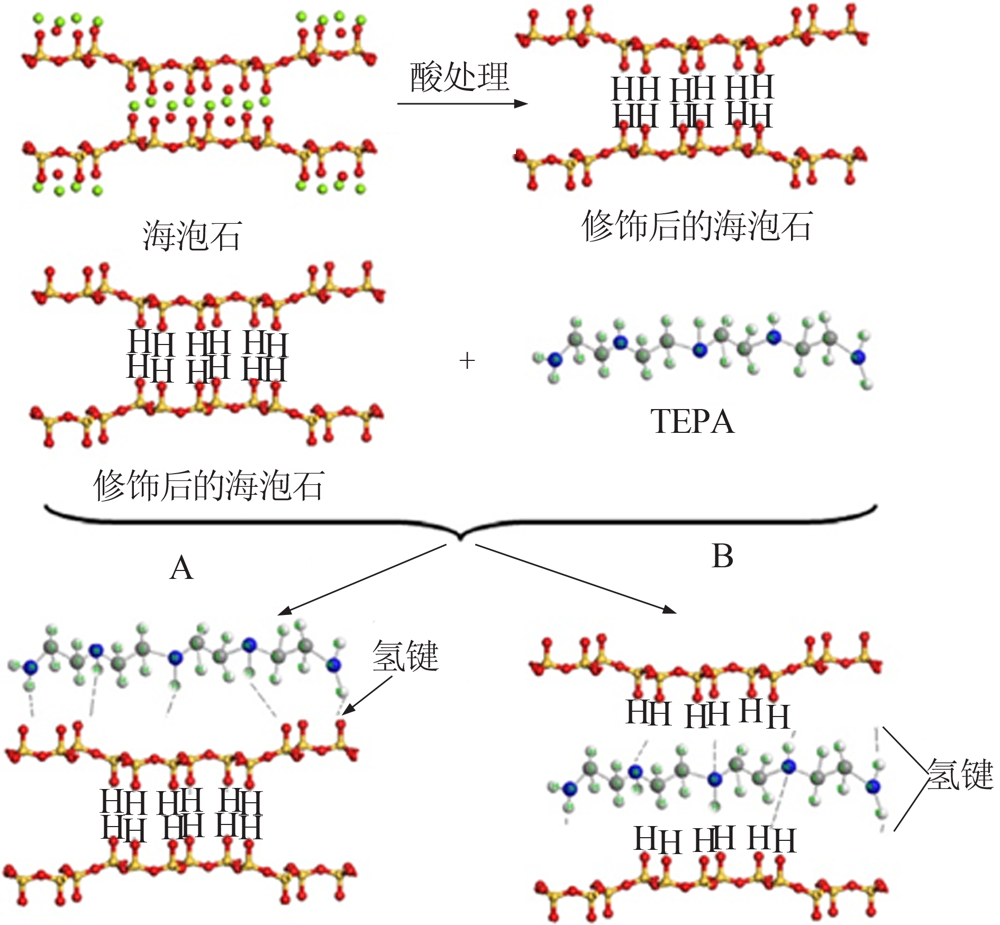
Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (12): 29-34.doi: 10.19964/j.issn.1006-4990.2021-0567
• Inorganic Noval Materials—Carbon Resources Conversion and Utilization • Previous Articles Next Articles
Research progress of inorganic solid adsorbents in carbon dioxide capture
GUO Wei1( ),SHI Han1,YUAN Biao2
),SHI Han1,YUAN Biao2
- 1. CNOOC China Limited Shenzhen Branch,Shenzhen 518052,China
2. CenerTech Tianjin Chemical Research and Design Institute Co.,Ltd.
-
Received:2021-09-17Online:2021-12-10Published:2021-12-16
CLC Number:
Cite this article
GUO Wei,SHI Han,YUAN Biao. Research progress of inorganic solid adsorbents in carbon dioxide capture[J]. Inorganic Chemicals Industry, 2021, 53(12): 29-34.
share this article
| [1] |
SZULEJKO J E, KUMAR P, DEEP A, et al. Global warming projec- tions to 2100 using simple CO2 greenhouse gas modeling and com- ments on CO2 climate sensitivity factor[J]. Atmospheric Pollution Research, 2017, 8(1):136-140.
doi: 10.1016/j.apr.2016.08.002 |
| [2] |
ANDERSON T R, HAWKINS E, JONES P D. CO2,the greenhouse effect and global warming:From the pioneering work of Arrhenius and Callendar to today′s Earth system models[J]. Endeavour, 2016, 40(3):178-187.
doi: 10.1016/j.endeavour.2016.07.002 |
| [3] | 王建行, 赵颖颖, 李佳慧, 等. 二氧化碳的捕集、固定与利用的研究进展[J]. 无机盐工业, 2020, 52(4):12-17. |
| [4] | BOOT-HANDFORD M E, ABANADES J C, ANTHONY E J, et al. Carbon capture and storage update[J]. Energy & Environmental Sci- ence, 2014, 7(1):130-189. |
| [5] |
KÄTELHÖN A, MEYS R, DEUTZ S, et al. Climate change mitigation potential of carbon capture and utilization in the chemical indust- ry[J]. Proceedings of the National Academy of Sciences, 2019, 116(23):11187-11194.
doi: 10.1073/pnas.1821029116 |
| [6] | BUI M, ADJIMAN C S, BARDOW A, et al. Carbon capture and stor- age(CCS):The way forward[J]. Energy & Environmental Science, 2018, 11(5):1062-1176. |
| [7] | HAIDER M B, HUSSAIN Z, KUMAR R. CO2 absorption and kinetic study in ionic liquid amine blends[J]. Journal of Molecular Li- quids, 2016, 224:1025-1031. |
| [8] |
PETERS L, HUSSAIN A, FOLLMANN M, et al. CO2 removal from natural gas by employing amine absorption and membrane technolo- gy-A technical and economical analysis[J]. Chemical Engineering Journal, 2011, 172(2):952-960.
doi: 10.1016/j.cej.2011.07.007 |
| [9] | VEAWAB A, TONTIWACHWUTHIKUL P, CHAKMA A. Corrosion behavior of carbon steel in the CO2 absorption process using aque- ous amine solutions[J]. Industrial & Engineering Chemistry Rese- arch, 1999, 38(10):3917-3924. |
| [10] |
HONG S-M, JANG E, DYSART A D, et al. CO2 capture in the su- stainable wheat-derived activated microporous carbon compart- ments[J]. Scientific Reports, 2016, 6(1):1-10.
doi: 10.1038/s41598-016-0001-8 |
| [11] | TRICKETT C A, HELAL A, AL-MAYTHALONY B A, et al. The chemistry of metal-organic frameworks for CO2 capture,regenera- tion and conversion[J]. Nature Reviews Materials, 2017, 2(8):1-16. |
| [12] | 柴彤, 赵瑞红, 栗明宏, 等. 氨基改性有序介孔氧化铝吸附二氧化碳性能研究[J]. 无机盐工业, 2016, 48(12):14-18. |
| [13] | 周静, 邹洪涛, 邢焰, 等. 磷石膏制备纳米氧化钙基二氧化碳吸附剂工艺的优化[J]. 无机盐工业, 2016, 48(10):73-76. |
| [14] |
KACEM M, PELLERANO M, DELEBARRE A. Pressure swing adsorption for CO2/N2 and CO2/CH4 separation:Comparison between activated carbons and zeolites performances[J]. Fuel Processing Technology, 2015, 138:271-283.
doi: 10.1016/j.fuproc.2015.04.032 |
| [15] |
GARCÍA S, GIL M V, MARTÍN C F, et al. Breakthrough adsorption study of a commercial activated carbon for pre-combustion CO2 capture[J]. Chemical Engineering Journal, 2011, 171(2):549-556.
doi: 10.1016/j.cej.2011.04.027 |
| [16] | BEN-MANSOUR R, HABIB M A, BAMIDELE O E, et al. Carbon capture by physical adsorption:Materials,experimental investiga- tions and numerical modeling and simulations-A review[J]. App- lied Energy, 2016, 161:225-255. |
| [17] |
ALABADI A, RAZZAQUE S, YANG Y, et al. Highly porous activat- ed carbon materials from carbonized biomass with high CO2 captur- ing capacity[J]. Chemical Engineering Journal, 2015, 281:606-612.
doi: 10.1016/j.cej.2015.06.032 |
| [18] |
BHATT P M, BELMABKHOUT Y, CADIAU A, et al. A fine-tuned fluorinated MOF addresses the needs for trace CO2 removal and air capture using physisorption[J]. Journal of the American Chemical Society, 2016, 138(29):9301-9307.
doi: 10.1021/jacs.6b05345 |
| [19] |
BELMABKHOUT Y, GUILLERM V, EDDAOUDI M. Low co- ncentration CO2 capture using physical adsorbents:Are metal-or- ganic frameworks becoming the new benchmark materials?[J]. Chemical Engineering Journal, 2016, 296:386-397.
doi: 10.1016/j.cej.2016.03.124 |
| [20] |
SAI BHARGAVA REDDY M, PONNAMMA D, SADASIVUNI K K, et al. Carbon dioxide adsorption based on porous materials[J]. RSC Advances, 2021, 11(21):12658-12681.
doi: 10.1039/D0RA10902A |
| [21] |
AZMI A A, AZIZ M A A. Mesoporous adsorbent for CO2 capture application under mild condition:A review[J]. Journal of Environ- mental Chemical Engineering, 2019, 7(2).Doi: 10.1016/j.jece.2019.103022.
doi: 10.1016/j.jece.2019.103022 |
| [22] |
LIU R S, SHI X D, WANG C T, et al. Advances in post-combus- tion CO2 capture by physical adsorption:From materials innovation to separation practice[J]. ChemSusChem, 2021, 14(6):1428-1471.
doi: 10.1002/cssc.v14.6 |
| [23] |
HAO G-P, LI W-C, QIAN D, et al. Structurally designed synjournal of mechanically stable poly(benzoxazine-co-resol)-based porous carbon monoliths and their application as high-performance CO2 capture sorbents[J]. Journal of the American Chemical Society, 2011, 133(29):11378-11388.
doi: 10.1021/ja203857g |
| [24] |
LIU Q, HE P, QIAN X, et al. Enhanced CO2 adsorption performance on hierarchical porous ZSM-5 zeolite[J]. Energy & Fuels, 2017, 31(12):13933-13941.
doi: 10.1021/acs.energyfuels.7b02543 |
| [25] |
KONGNOO A, TONTISIRIN S, WORATHANAKUL P, et al. Sur- face characteristics and CO2 adsorption capacities of acid-activated zeolite 13X prepared from palm oil mill fly ash[J]. Fuel, 2017, 193:385-394.
doi: 10.1016/j.fuel.2016.12.087 |
| [26] | BARTHOMEUF D. Framework induced basicity in zeolites[J]. Mi- croporous and Mesoporous Materials, 2003, 66(1):1-14. |
| [27] |
BARTHOMEUF D. Conjugate acid-base pairs in zeolites[J]. The Journal of Physical Chemistry, 1984, 88(1):42-45.
doi: 10.1021/j150645a010 |
| [28] |
WALTON K S, ABNEY M, BDOUGLAS LEVAN M. CO2 ad- sorption in Y and X zeolites modified by alkali metal cation exch- ange[J]. Microporous and Mesoporous Materials, 2006, 91(1):78-84.
doi: 10.1016/j.micromeso.2005.11.023 |
| [29] |
PHAM T D, HUDSON M R, BROWN C M, et al. On the structure- property relationships of cation-exchanged ZK-5 zeolites for CO2 adsorption[J]. ChemSusChem, 2017, 10(5):946-957.
doi: 10.1002/cssc.v10.5 |
| [30] | YANG J, SHANG H, KRISHNA R, et al. Adjusting the proportions of extra-framework K+ and Cs+ cations to construct a“molecular gate” on ZK-5 for CO2 removal[J]. Microporous and Mesoporous Materi- als, 2018, 268:50-57. |
| [31] | SUN M, GU Q, HANIF A, et al. Transition metal cation-exchanged SSZ-13 zeolites for CO2 capture and separation from N2[J]. Che- mical Engineering Journal, 2019, 370:1450-1458. |
| [32] |
SU F, LU C, KUO S-C, et al. Adsorption of CO2 on amine-functio- nalized Y-type zeolites[J]. Energy & Fuels, 2010, 24(2):1441-1448.
doi: 10.1021/ef901077k |
| [33] |
MURGE P, DINDA S, ROY S. Zeolite-based sorbent for CO2 capture:Preparation and performance evaluation[J]. Langmuir, 2019, 35(46):14751-14760.
doi: 10.1021/acs.langmuir.9b02259 |
| [34] | WANG Y, DU T, QIU Z, et al. CO2 adsorption on polyethylenimine- modified ZSM-5 zeolite synthesized from rice husk ash[J]. Materi- als Chemistry and Physics, 2018, 207:105-113. |
| [35] |
CHEN C, KIM S-S, CHO W-S, et al. Polyethylenimine-incorpora- ted zeolite 13X with mesoporosity for post-combustion CO2 cap- ture[J]. Applied Surface Science, 2015, 332:167-171.
doi: 10.1016/j.apsusc.2015.01.106 |
| [36] | MADDEN D, CURTIN T. Carbon dioxide capture with amino- functionalised zeolite-β:A temperature programmed desorption study under dry and humid conditions[J]. Microporous and Meso- porous Materials, 2016, 228:310-317. |
| [37] |
SANZ R, CALLEJA G, ARENCIBIA A, et al. Amino functionalized mesostructured SBA-15 silica for CO2 capture:Exploring the rela- tion between the adsorption capacity and the distribution of amino groups by TEM[J]. Microporous and Mesoporous Materials, 2012, 158:309-317.
doi: 10.1016/j.micromeso.2012.03.053 |
| [38] |
SON W-J, CHOI J-S, AHN W-S. Adsorptive removal of carbon dioxide using polyethyleneimine-loaded mesoporous silica materi- als[J]. Microporous and Mesoporous Materials, 2008, 113(1):31-40.
doi: 10.1016/j.micromeso.2007.10.049 |
| [39] | JAHANDAR L M, SAYARI A. CO2 capture using triamine-gra- fted SBA-15:The impact of the support pore structure[J]. Chemi- cal Engineering Journal, 2018, 334:1260-1269. |
| [40] |
SANZ-PÉREZ E S, ARENCIBIA A, CALLEJA G, et al. Tuning the textural properties of HMS mesoporous silica.Functionalization to- wards CO2 adsorption[J]. Microporous and Mesoporous Materials, 2018, 260:235-244.
doi: 10.1016/j.micromeso.2017.10.038 |
| [41] |
SUBAGYONO D J N, LIANG Z, KNOWLES G P, et al. Amine mo- dified mesocellular siliceous foam (MCF) as a sorbent for CO2[J]. Chemical Engineering Research and Design, 2011, 89(9):1647-1657.
doi: 10.1016/j.cherd.2011.02.019 |
| [42] | D′ALESSANDRO D M, SMIT BLONG J R. Carbon dioxide capture:Prospects for new materials[J]. Angewandte Chemie In- ternational Edition, 2010, 49(35):6058-6082. |
| [43] |
IRANI M, FAN M, ISMAIL H, et al. Modified nanosepiolite as an inexpensive support of tetraethylenepentamine for CO2 sorption[J]. Nano Energy, 2015, 11:235-246.
doi: 10.1016/j.nanoen.2014.11.005 |
| [44] | CECILIA J A, VILARRASA-GARCíA E, CAVALCANTE C L, et al. Evaluation of two fibrous clay minerals(sepiolite and palygors- kite) for CO2 Capture[J]. Journal of Environmental Chemical En- gineering, 2018, 6(4):4573-4587. |
| [45] |
WANG W, XIAO J, WEI X, et al. Development of a new clay sup- ported polyethylenimine composite for CO2 capture[J]. Applied Energy, 2014, 113:334-341.
doi: 10.1016/j.apenergy.2013.03.090 |
| [46] |
GÓMEZ-POZUELO G, SANZ-PÉREZ E S, ARENCIBIA A, et al. CO2 adsorption on amine-functionalized clays[J]. Microporous and Mesoporous Materials, 2019, 282:38-47.
doi: 10.1016/j.micromeso.2019.03.012 |
| [47] | VILARRASA-GARCÍA E, CECILIA J A, AZEVEDO D C S, et al. Evaluation of porous clay heterostructures modified with amine species as adsorbent for the CO2 capture[J]. Microporous and Me- soporous Materials, 2017, 249:25-33. |
| [48] |
WANG J, HEERWIG A, LOHE M R, et al. Fungi-based porous carbons for CO2 adsorption and separation[J]. Journal of Materials Chemistry, 2012, 22(28):13911-13913.
doi: 10.1039/c2jm32139d |
| [49] |
TIAN W, ZHANG H, SUN H, et al. Heteroatom(N or N-S)-doping induced layered and honeycomb microstructures of porous carbons for CO2 capture and energy applications[J]. Advanced Functional Materials, 2016, 26(47):8651-8661.
doi: 10.1002/adfm.v26.47 |
| [50] |
GUO Y, TAN C, SUN J, et al. Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption[J]. Chemical En- gineering Journal, 2020, 381.Doi: 10.1016/j.cej.2019.122736.
doi: 10.1016/j.cej.2019.122736 |
| [51] | SEVILLA M, FUERTES A B. Sustainable porous carbons with a superior performance for CO2 capture[J]. Energy & Environmen- tal Science, 2011, 4(5):1765-1771. |
| [52] |
WANG H, XU C, ZHOU Y, et al. Fabrication of hierarchical N-do- ped carbon nanotubes for CO2 adsorption[J]. Nano, 2019, 14(6).Doi: 10.1142/S1793292019500723.
doi: 10.1142/S1793292019500723 |
| [53] |
MISHRA A K, RAMAPRABHU S. Carbon dioxide adsorption in graphene sheets[J]. AIP Advances, 2011, 1(3).Doi: 10.1063/1.3638178.
doi: 10.1063/1.3638178 |
| [1] | WANG Minrui, TIAN Guiying, ZHANG Ao, GE Junjie, ZHANG Lei, XIANG Jun, TANG Na. Study on granulation optimization for Al-based lithium adsorbent and its lithium recovery performance from brine [J]. Inorganic Chemicals Industry, 2025, 57(3): 36-42. |
| [2] | LIU Xinlong, YANG Zhenyu, HAO He, LIU Shuxin, WU Chenyang, WANG Xingli, MA Qingqing. Study on shaped 4A zeolite synthesized with aluminum extraction residue by fly ash [J]. Inorganic Chemicals Industry, 2025, 57(3): 78-85. |
| [3] | GUO Yingjun, WU Songsong, DING Chunyan, ZHAO Shikai, SONG Tao, WEN Guangwu. Preparation of SSZ-13 zeolite membrane from glass-ceramics-strontium feldspar by crystal transformation method [J]. Inorganic Chemicals Industry, 2025, 57(2): 76-82. |
| [4] | FAN Jingxin, LI Bin, HONG Luwei, HONG Meihua, GONG Xin. Research status and prospects of olefin removal catalyst from aromatic reforming oil [J]. Inorganic Chemicals Industry, 2025, 57(2): 14-25. |
| [5] | SHEN Xiaoqian, ZHOU Fei, LIU Wanchen, XU Lu, WU Junshu. Study on synthesis of FeS modified calcium silicate hydrate composites and their total Cr removal performance [J]. Inorganic Chemicals Industry, 2025, 57(2): 57-67. |
| [6] | ZHU Jicheng, YANG Qixin, LIANG Haoquan, WANG Zengkun, OUYANG Fugui, DI Jing, GAI Xikun. Effect of confined catalyst Ni@S2 on performance of methane dry reforming reaction [J]. Inorganic Chemicals Industry, 2025, 57(2): 138-146. |
| [7] | LIU Huangfei, ZHANG Li, LIU Tao. Research progress of fast synthesis technologies of zeolites [J]. Inorganic Chemicals Industry, 2025, 57(2): 36-43. |
| [8] | WANG Jianjie, SHU Xiaolong, XIAO Xia, WANG Peng, FAN Xiaoqiang, KONG Lian, XIE Zean, ZHAO Zhen. Study on synthesis of hierarchical flower⁃like ZSM-5 zeolite and its catalytic performance for n-octane cracking [J]. Inorganic Chemicals Industry, 2024, 56(8): 139-146. |
| [9] | WANG Ting, ZHANG Wenwen, MAO Qing, LÜ Li, LIU Changzhen. Research progress of catalytic system and materials for electrocatalytic reduction of carbon dioxide to ethanol [J]. Inorganic Chemicals Industry, 2024, 56(7): 1-10. |
| [10] | LIU Min, HUANG Xiu, ZHANG Liyuan. Research progress of S-type heterojunction photocatalysts [J]. Inorganic Chemicals Industry, 2024, 56(7): 18-27. |
| [11] | LIU Qingcui, LI Yunqing, PANG Ruiqi, TIAN Yaping, CHEN Yiying, LI Fang, LI Qiming. Preparation of Zn/Co-ZIF derived porous carbon supported Pd as catalyst and its application to formic acid dehydrogenation [J]. Inorganic Chemicals Industry, 2024, 56(6): 147-152. |
| [12] | SHEN Haiyan, LI Fangqin, REN Jianxing, WU Jiang, GUAN Zhenzhen, PAN Weiguo. Research progress on chemical absorption method for capturing carbon dioxide [J]. Inorganic Chemicals Industry, 2024, 56(5): 11-19. |
| [13] | PEI Xiaogang, ZHANG Peng, DONG Shanshan, GE Shanshan, ZHAO Yuelong. Hydroxyapatite composite humic acid and its removal of Mn(Ⅱ) [J]. Inorganic Chemicals Industry, 2024, 56(5): 70-77. |
| [14] | DI Lu, WANG Weiguo, CHEN Juexian, WU Chuanshu. Study on preparation of transition metal-supported Silicalite-1 zeolite catalyst and its catalytic performance for furfural hydrogenation [J]. Inorganic Chemicals Industry, 2024, 56(4): 125-132. |
| [15] | ZHAO Chuang, CHEN Zihao, ZHANG Boyu, LI Ben, JIN Fengying, LI Bin, SUN Zhenhai, GUO Chunlei. Study on adsorption and separation performance of molecular sieve adsorbents for different types of diesel [J]. Inorganic Chemicals Industry, 2024, 56(3): 80-85. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||