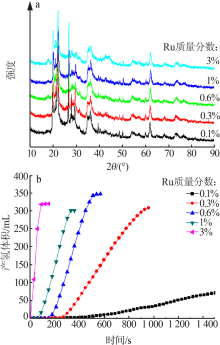
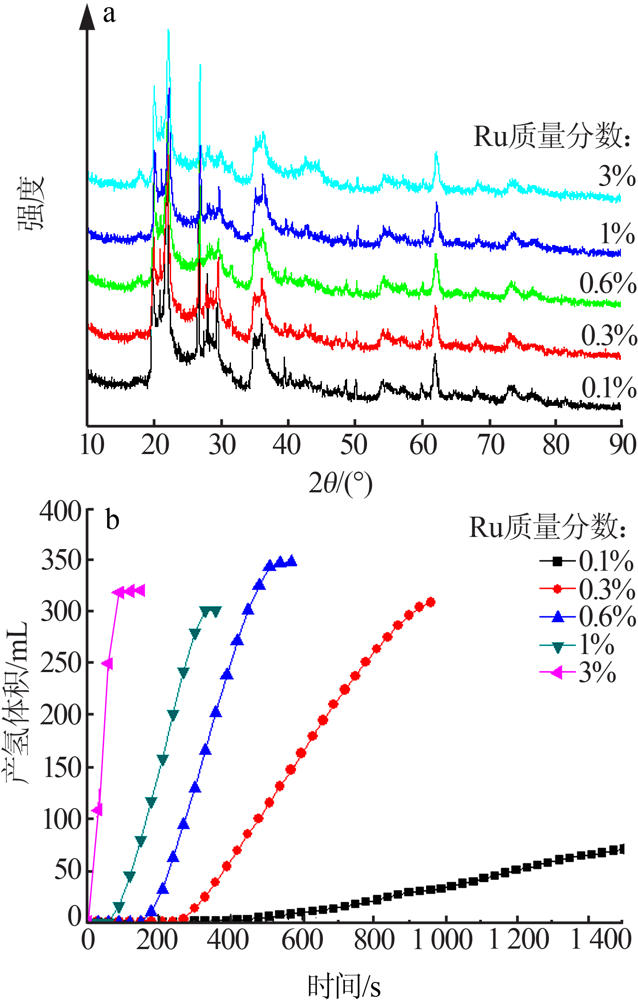
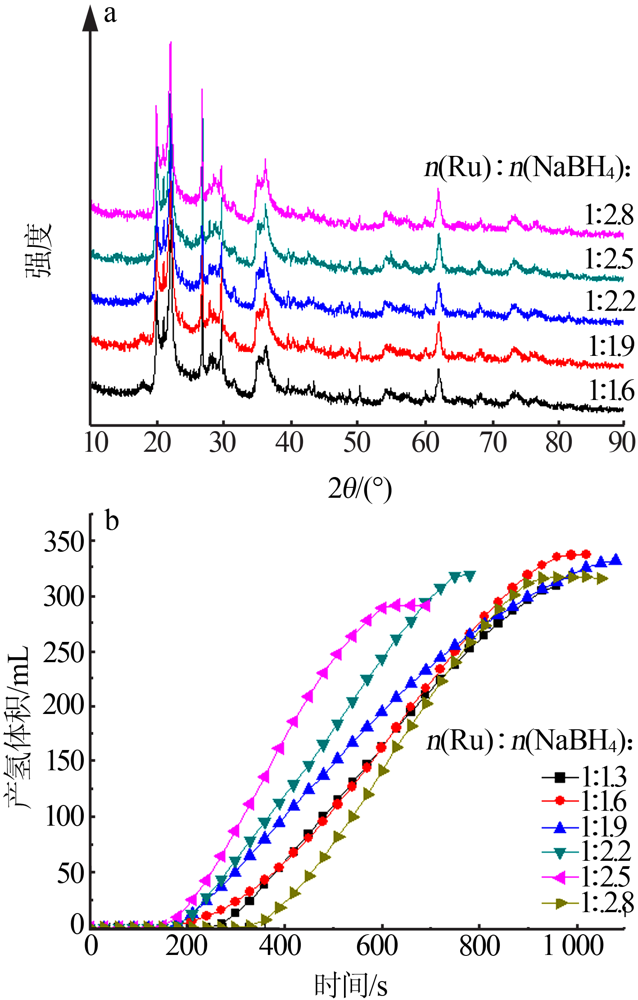
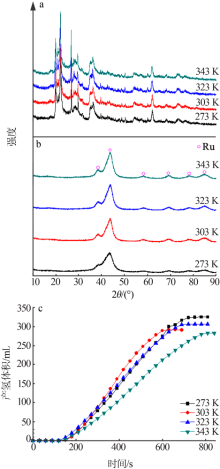
Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (6): 199-204.doi: 10.19964/j.issn.1006-4990.2020-0421
• Catalytic Materials • Previous Articles Next Articles
Study on preparation of bentonite supported Ru catalysts and their performance for hydrogen generation from catalytic hydrolysis of ammonia borane
Chen Lingxia1( ),Wang Qi1,Sun Haijie1(
),Wang Qi1,Sun Haijie1( ),Chen Zhihao2,Liu Ranran1
),Chen Zhihao2,Liu Ranran1
- 1. School of Chemistry and Chemical Engineering,Zhengzhou University,Zhengzhou 450044,China
2. Zhengzhou Tobacco Research Institute of CNTC
-
Received:2020-07-25Online:2021-06-10Published:2021-07-08 -
Contact:Sun Haijie E-mail:chenlingxia2120@vip.163.com;sunhaijie406@163.com
CLC Number:
Cite this article
Chen Lingxia,Wang Qi,Sun Haijie,Chen Zhihao,Liu Ranran. Study on preparation of bentonite supported Ru catalysts and their performance for hydrogen generation from catalytic hydrolysis of ammonia borane[J]. Inorganic Chemicals Industry, 2021, 53(6): 199-204.
share this article
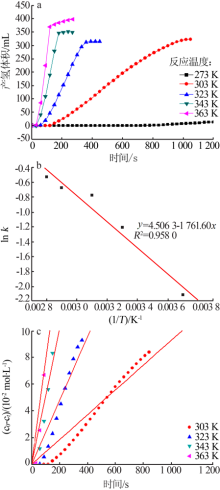
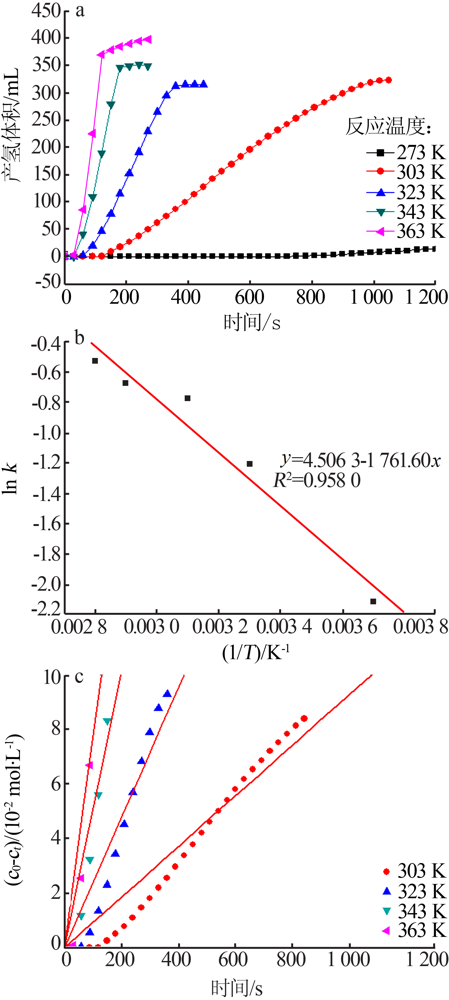
Table 1
Apparent activation energy of Ru catalysts supported with different supports for hydrolysis of NH3BH3towards hydrogen generation "
| 催化剂 | 表观活化能/ (kJ·mol-1) | 催化剂 | 表观活化能/ (kJ·mol-1) |
|---|---|---|---|
| Ru/g-C3N4[ | 37 | Ru/MIL-96[ | 48 |
| Ru/C[ | 35 | Ru/MIL-101[ | 51 |
| Ru/Graphene[ | 12 | Ru/micro HAP[ | 58 |
| Ru/Carbon[ | 76 | Ru/TiO2[ | 87 |
| Ru/γ-Al2O3[ | 67 | Ru/Ce(OH)CO3[ | 60 |
| Ru/Al2O3[ | 48 | Ru/HAP[ | 44 |
| Ru/MIL-41[ | 42 | Ru/Ben(本研究) | 15 |
| Ru/MIL-53[ | 29 |
| [1] | 聂文丹, 杨齐凤, 卢章辉. 介孔硅负载超细Pd纳米粒子催化甲酸分解脱氢[J]. 江西师范大学学报:自然科学版, 2019,43(4):416-424. |
| [2] | 孙海杰, 陈凌霞, 张玉凤, 等. 钴-硼/二氧化锆催化剂催化硼氢化钠水解制氢研究[J]. 无机盐工业, 2019,51(3):72-76. |
| [3] | 孙海杰, 陈凌霞, 黄振旭, 等. 第四周期过渡金属催化硼氢化钠水解制氢研究[J]. 无机盐工业, 2017,49(5):14-17. |
| [4] | 孙海杰, 黄振旭, 王雅苹, 等. 非晶态合金Ru-B/ZrO2催化剂催化硼氢化钠水解制氢性能的研究[J]. 化工新型材料, 2018,46(1):102-105. |
| [5] | 张帅, 王斯瑶, 姜召, 等. 静电纺丝技术在氨硼烷水解脱氢催化剂制备中的应用[J]. 化工进展, 2019,38(7):3194-3206. |
| [6] | 杨晓婧, 尚伟, 李兰兰, 等. 金属催化氨硼烷制氢研究进展[J]. 电源技术, 2014,38(7):1387-1389. |
| [7] | 桑婉璐, 李兰兰, 高若源, 等. 氨硼烷水解制氢催化剂载体的研究进展[J]. 材料导报, 2017,31(9):27-33. |
| [8] | 刘仲毅, 孙海杰, 王东斌, 等. 纳米ZrO2作分散剂的Ru-Zn催化剂上苯选择加氢制环己烯[J]. 催化学报, 2010,31(2):150-152. |
| [9] | 姚鹏泉, 祝琳华, 司甜. 从天然膨润土制备钠基蒙脱石催化剂载体[J]. 非金属矿, 2013,36(1):24-26. |
| [10] | Wang W T, Liu H Z, Wu T B, et al. Ru catalyst supported on ben-tonite for partial hydrogenation of benzene to cyclohexene[J]. Jo-urnal of Molecular Catalysis A:Chemical, 2012,355:174-179. |
| [11] | 周雷雷, 张荣斌, 张婷婷, 等. 钐对Ni/膨润土催化性能的改性研究[J]. 非金属矿, 2013,37(2):27-29. |
| [12] | 孙海杰, 陈凌霞, 陈秀丽. ZrO2织构性质对Ru-B/ZrO2催化剂的结构及其苯选择加氢性能的影响[J]. 石油化工, 2015,44(9):1066-1070. |
| [13] | 孙海杰, 陈凌霞, 黄振旭, 等. Ru-Zn催化剂在苯选择加氢制环己烯反应中的粒径效应[J]. 高等学校化学学报, 2015,36(10):1969-1976. |
| [14] |
Cao N, Luo W, Cheng G Z. One-step synjournal of graphene suppor-ted Ru nanoparticles as efficient catalysts for hydrolytic dehydro-genation of ammonia borane[J]. International Journal of Hydrogen Energy, 2013,38(27):11964-11972.
doi: 10.1016/j.ijhydene.2013.06.125 |
| [15] |
Basu S, Brockman A, Gagare P, et al. Chemical kinetics of Ru-catalyzed ammonia borane hydrolysis[J]. Journal of Power Sources, 2009,188(1):238-243.
doi: 10.1016/j.jpowsour.2008.11.085 |
| [16] |
Giovanni P R, Umit B D, Philippe M, et al. Faicile synjournal by po-lyol method of a ruthenium catalyst supported on γ-Al2O3 for hy-drolytic dehydrogenation of ammonia borane[J]. Catalysis Today, 2011,170(1):85-92.
doi: 10.1016/j.cattod.2011.01.040 |
| [17] | Can H, Metin Ö. A facile synjournal of early monodisperse ruthenium nanoparticles and their catalysis in the hydrolytic dehydrogenation of ammoniaborane for chemical hydrogen storage[J]. Applied Ca-talysis B:Environmental, 2012,125(3):304-310. |
| [18] |
Akbayrak S, Erdek P, Özkar S. Hydroxyapatite supported rutheni-um(0)nanoparticles catalyst in hydrolytic dehydrogenation of ammonia borane:Insight to the nanoparticles formation and hydro-gen evolution kinetics[J]. Applied Catalysis B:Environmental, 2013,142-143:187-195.
doi: 10.1016/j.apcatb.2013.05.015 |
| [19] | 孙海杰, 陈秀丽, 黄振旭, 等. NaOH浓度对苯选择加氢制环己烯Ru-Zn催化剂性能的影响[J]. 化工学报, 2016,67(4):1324-1332. |
| [20] |
Hu S, Chen Y W. Partial hydrogenation of benzene to cyclohexene on ruthenium catalysts supported on La2O3-ZnO binary oxides[J]. Industrial & Engineering Chemistry Research, 1997,36(12):5153-5159.
doi: 10.1021/ie970300y |
| [21] | 朱玉玲, 郑修成, 刘蒲. 磁性花生壳负载钯催化剂的制备及催化氨硼烷释氢性能[J]. 信阳师范学院学报:自然科学版, 2019,32(2):276-280. |
| [22] |
Dong H, Yang H, Ai X, et al. Hydrogen production from catalytic hydrolysis of sodium borohydride solution using nickel boride ca-talyst[J]. International Journal Hydrogen Energy, 2003,28(10):1095-1100.
doi: 10.1016/S0360-3199(02)00235-5 |
| [23] | 卢章辉, 杨宇雯, 陈祥树. 一种用于氨硼烷水解制氢的Ni-CeO2@graphene复合纳米催化剂及其制备方法:中国,103990465B [P]. 2016-10-05. |
| [24] | Liang H Y, Chen G Z, Desinan S, et al. In situ facile synjournal of ruthenium nanocluster catalyst supported on carbon black for hy-drogen generation from the hydrolysis of ammonia-borane[J]. In-ternational Journal of Hydrogen Energy, 2012,37(23):17921-17927. |
| [25] |
Park S, An J, Potts J R, et al. Hydrazine-reduction of graphite-and graphene oxide[J]. Carbon, 2011,49(9):3019-3023.
doi: 10.1016/j.carbon.2011.02.071 |
| [26] |
Yang K Z, Zhou L Q, Yu G F, et al. Ru nanoparticles supported on MIL-53(Cr,Al) as efficient catalysts for hydrogen generation from hydrolysis of ammonia borane[J]. International Journal of Hydrogen Energy, 2016,41(15):6300-6309.
doi: 10.1016/j.ijhydene.2016.02.104 |
| [27] | Wen L, Su J, Wu X J, et al. Ruthenium supported on MIL-96:An efficient catalyst for hydrolytic dehydrogenation of ammonia borane for chemical hydrogen storage[J]. International Journal of Hydro-gen Energy, 2014,39(30):17129-17135. |
| [28] |
Cao N, Liu T, Su J, et al. Ruthenium supported on MIL-101 as an efficient catalyst for hydrogen generation from hydrolysis of amine boranes[J]. New Journal of Chemistry, 2014,38(9):4032-4035.
doi: 10.1039/C4NJ00739E |
| [29] |
Kohsuke M, Kohei M, Hiromi Y, et al. Ru and Ru-Ni nanoparticles on TiO2 support as extremely active catalysts for hydrogen produc-tion from ammonia-borane[J]. ACS Catalysis, 2016,6(5):3128-3135.
doi: 10.1021/acscatal.6b00715 |
| [30] | 陈健民, 卢章辉, 熊丽华. Ru/Ce(OH)CO3纳米复合材料催化氨硼烷水解产氢[J]. 无机化学学报, 2016,32(10):1816-1824. |
| [31] | 孙海杰, 刘欣改, 陈志浩, 等. 羟基磷灰石负载Ru催化氨硼烷产氢性能研究[J]. 江西师范大学学报:自然科学版, 2020,44(4):424-428. |
| [1] | BAI Chaopeng, PENG Hui, CHEN Yuting, LUO Jiang, ZHANG Shengming. Research progress of recovery,purification and recycling of ruthenium from scrap ruthenium materials [J]. Inorganic Chemicals Industry, 2024, 56(4): 24-33. |
| [2] | ZHOU Xuan, LI Mengrui, CHEN Yichen, FAN Huiqiang, WANG Bin, YUAN Gang. Research progress of nickel-based phosphide composites in improving of catalytic water electrolysis for hydrogen evolution performance [J]. Inorganic Chemicals Industry, 2024, 56(4): 8-15. |
| [3] | WANG Ruting, ZHAO Xiaorong, HUANG Xuquan, WANG Haojie, XUE Fei, CAI Jiawei. Research on preparation and early performance of mixed phase phosphogypsum-based cementing materials [J]. Inorganic Chemicals Industry, 2024, 56(3): 98-104. |
| [4] | WU Bingdang, PAN Yinglu, ZHAO Qinzheng, WU Wei, XU Xiaoyi, HUANG Tianyin. Preparation of novel lanthanum coagulants and their deep phosphorus removal properties in low temperature and turbidity water [J]. Inorganic Chemicals Industry, 2024, 56(2): 127-135. |
| [5] | LI Qiang, YOU Xiaomin, SHE Xuefeng, JIANG Zeyi, XUE Qingguo, WANG Jingsong. Effect of calcination temperature and carbon structure on compressive strength of CaO-containing carbon pellets [J]. Inorganic Chemicals Industry, 2023, 55(9): 43-49. |
| [6] | YUAN Jinghua, WU Junhu, YANG XiuShan, XU Dehua, ZHANG Zhiye. Research on process of production ammonium dihydrogen phosphate by ammonia purification of nitro-phosphoric acid [J]. Inorganic Chemicals Industry, 2023, 55(9): 50-56. |
| [7] | YU Hongchao, ZHANG Mengmeng, JIN Tianxiang. Research progress of microstructure and crystal surface effect of Ag3PO4 photocatalysts [J]. Inorganic Chemicals Industry, 2023, 55(8): 13-20. |
| [8] | XU Li, ZHANG Qiang. Experimental study on properties of iron tailings powder cement-based materials [J]. Inorganic Chemicals Industry, 2023, 55(6): 116-123. |
| [9] | LI Zhiqiang, GUO Zhenghua, HE Yuru. Effect of Zr/Mn element substitution and annealing treatment on electrochemical properties of hydrogen storage alloys for automotive batteries [J]. Inorganic Chemicals Industry, 2023, 55(6): 78-84. |
| [10] | JIA Yuhong, HU Zhongpan, WANG Kunyuan, HAN Jingfeng, WEI Yingxu, LIU Zhongmin. Co anchored on silanol nests of S-1 zeolite for propane dehydrogenation to propylene [J]. Inorganic Chemicals Industry, 2023, 55(5): 121-127. |
| [11] | WANG Haosen, REN Bingchen, XU Dehua, YANG Xiushan, ZHANG Zhiye. Leaching process and kinetics of phosphorus-potassium ore decomposition by nitric acid [J]. Inorganic Chemicals Industry, 2023, 55(5): 45-51. |
| [12] | WEI Fengdan, ZHOU Huan, XIA Panping, ZHAO Yun. Boron species transformation and distribution law of Mg(BO2)2decomposed in LiCl aqueous solution [J]. Inorganic Chemicals Industry, 2023, 55(4): 45-53. |
| [13] | CHEN Tao, GAO Huimin. Study on preparation of phosphorus trichloride by microreaction [J]. Inorganic Chemicals Industry, 2023, 55(4): 72-75. |
| [14] | LI Liangrong, YANG Xiaozhe, CHEN Chuxin, LIU Yan, ZHANG Mengling, DING Yonghong. Research progress of photocatalytic water splitting of semiconductor core-shell materials for hydrogen production [J]. Inorganic Chemicals Industry, 2023, 55(3): 10-20. |
| [15] | WEN Yuan, ZHOU Chenliang, ZHANG Qiang, YU Linfei, HE Wenxiu, LIU Quansheng. Research progress of electronic and structure promoters of iron-based catalysts for FTO reaction [J]. Inorganic Chemicals Industry, 2023, 55(3): 36-46. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||