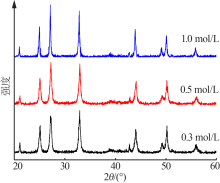
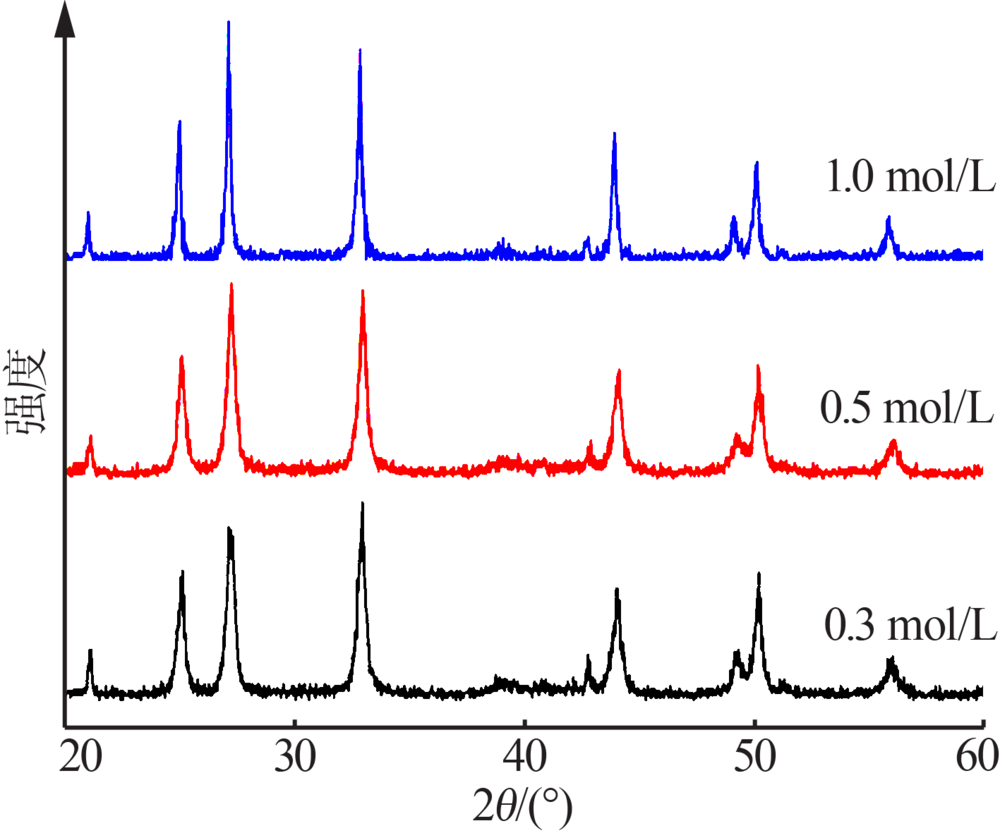
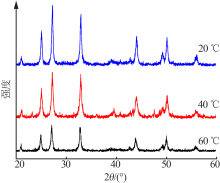
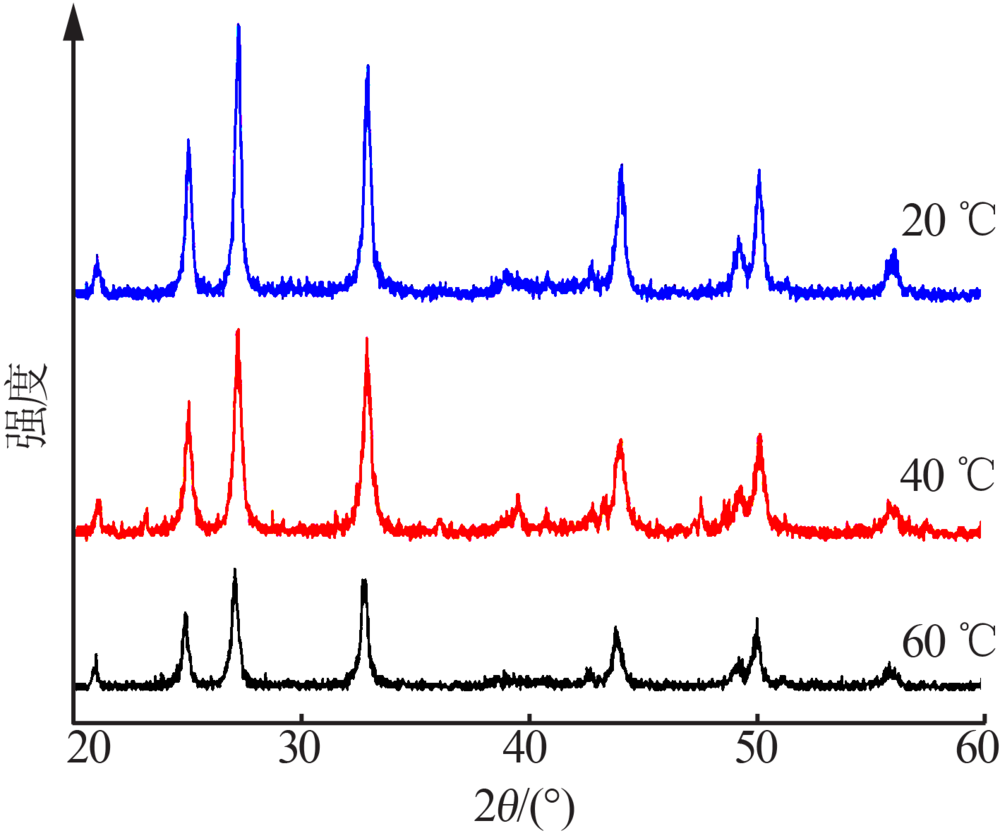
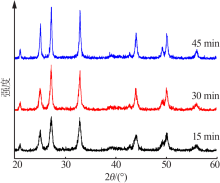
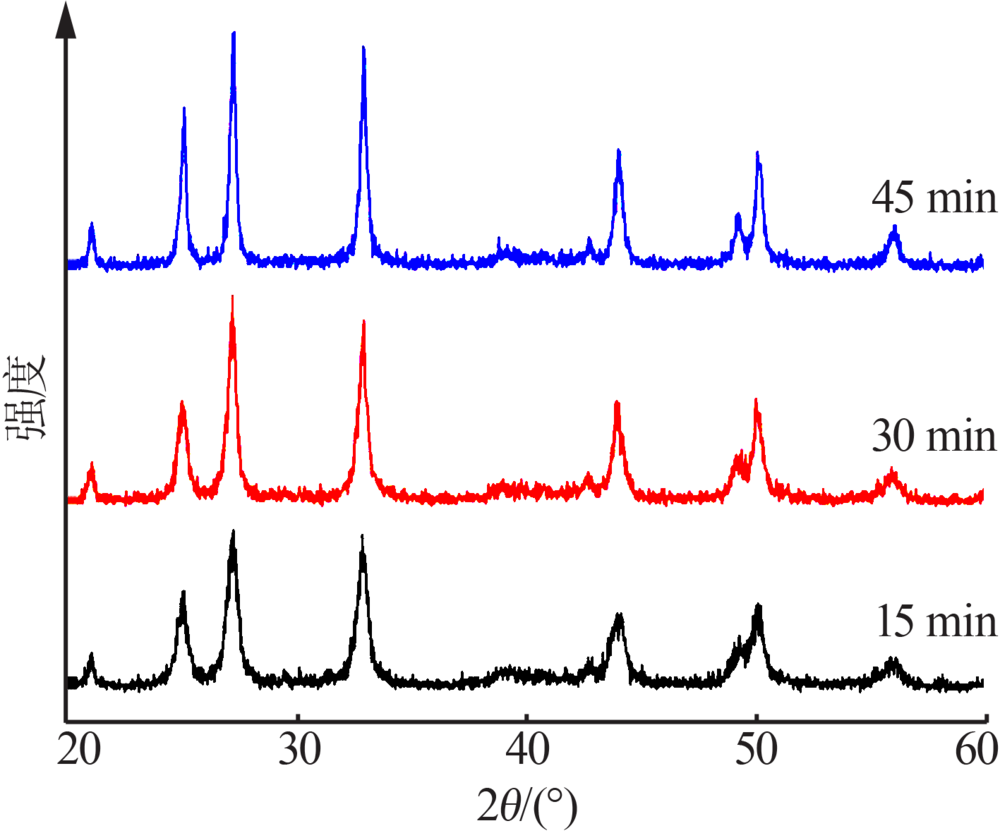
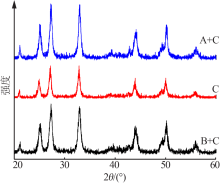
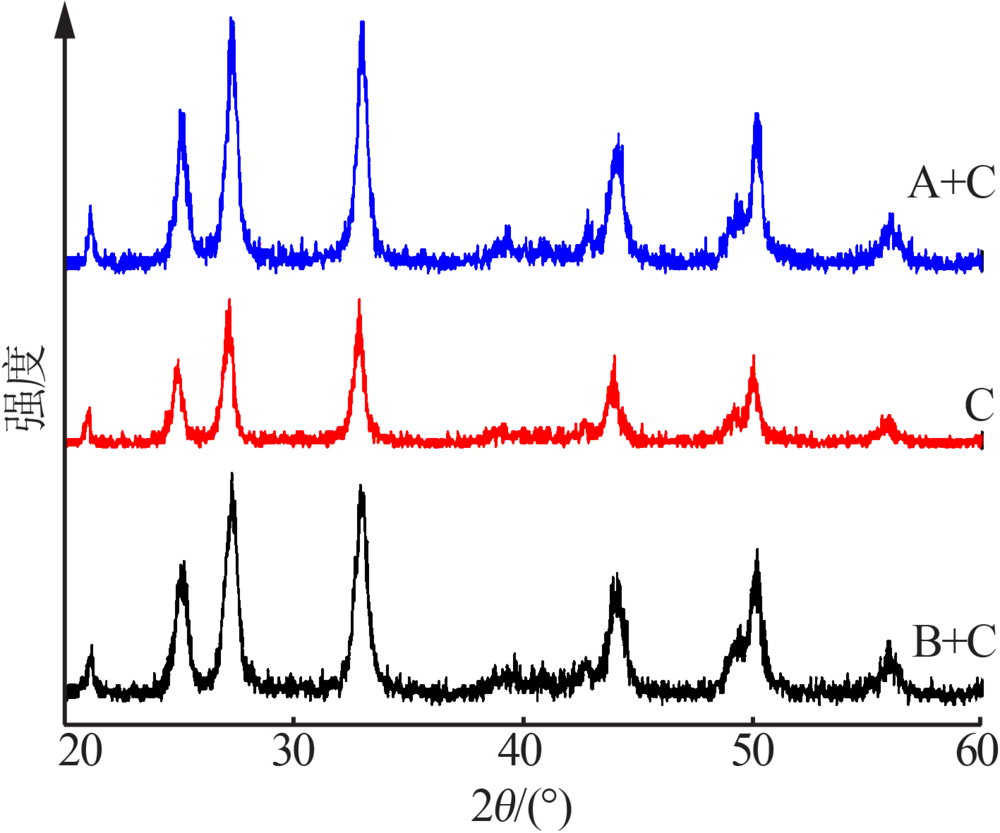
| [1] |
Cai G B, Zhao G X, Wang X K, et al. Synjournal of polyacrylic acid stabilized amorphous calcium carbonate nanoparticles and their application for removal of toxic heavy metal ions in water[J]. The Journal of Physical Chemistry C, 2010,114(30):12948-12954.
|
| [2] |
Mori Y, Enomae T, Isogai A. Application of vaterite-type calcium carbonate prepared by ultrasound for ink jet paper[J]. Journal of Imaging Science and Technology, 2010,54(2):020504.
|
| [3] |
Elert K, Herrera A, Cardell C. Pigment-binder interactions in calcium-based tempera paints[J]. Dyes and Pigments, 2018,148:236-248.
|
| [4] |
Zhao D, Wang C Q, Zhuo R X, et al. Modification of nanostructured calcium carbonate for efficient gene delivery[J]. Colloids Surf B Biointerfaces, 2014,118:111-116.
pmid: 24732398
|
| [5] |
Tang J, Sun D M, Qian W Y, et al. One-step bulk preparation of calcium carbonate nanotubes and its application in anticancer drug delivery[J]. Biological Trace Element Research, 2012,147(12/3):408-417.
|
| [6] |
Som A, Raliya R, Tian L, et al. Monodispersed calcium carbonate nanoparticles modulate local pH and inhibit tumor growth in vivo[J]. Nanoscale, 2016,8(25):12639-12647.
doi: 10.1039/c5nr06162h
pmid: 26745389
|
| [7] |
Bityutskii N P, Yakkonen K L, Petrova A I, et al. Calcium carbonate reduces the effectiveness of soil-added monosilicic acid in cucumber plants[J]. Journal of Soil Science and Plant Nutrition, 2019,19:660-670.
|
| [8] |
申争辉. 纳米碳酸钙在橡胶行业中的应用[J]. 化工新型材料, 2003(2):51-53.
|
| [9] |
明杏芬, 明晓东. 常规分散纳米碳酸钙对混凝土性能的影响研究[J]. 公路交通科技, 2019(6):25-30.
|
| [10] |
李增杰, 杨仲尼, 苏聚卿. 有机化改性纳米碳酸钙对沥青抗老化性能的影响研究[J]. 公路, 2018(11):259-264.
|
| [11] |
Naka K, Tanaka Y, Chujo Y. Effect of anionic starburst dendrimers on the crystallization of CaCO3 in aqueous solution:size control of spherical vaterite particles[J]. Langmuir, 2002,18(9):3655-3658.
|
| [12] |
Trushina D B, Bukreeva T V, Antipina M N. Size-controlled syn-journal of vaterite calcium carbonate by the mixing method:Aiming for nanosized particles[J]. Crystal Growth & Design, 2016,16(3):1311-1319.
|
| [13] |
Volodkin D V, Petrov A I, Prevot M, et al. Matrix polyelectrolyte microcapsules:new system for macromolecule encapsulation[J]. Langmuir, 2004,20(8):3398-3406.
doi: 10.1021/la036177z
pmid: 15875874
|
| [14] |
陈银霞, 纪献兵. 复分解法原位合成疏水球状球霰石纳米碳酸钙[J]. 无机盐工业, 2014,46(4):25-28.
|
| [15] |
GB/T 19281—2014 碳酸钙分析方法[S].
|
 ),Zhang Huijie1,Wang Shanshan2,Xue Yongqiang1,2(
),Zhang Huijie1,Wang Shanshan2,Xue Yongqiang1,2( )
)