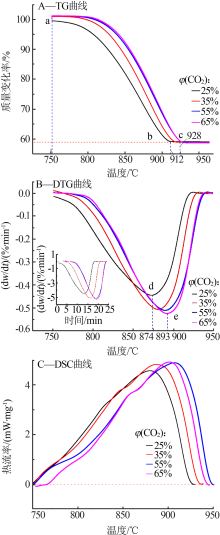
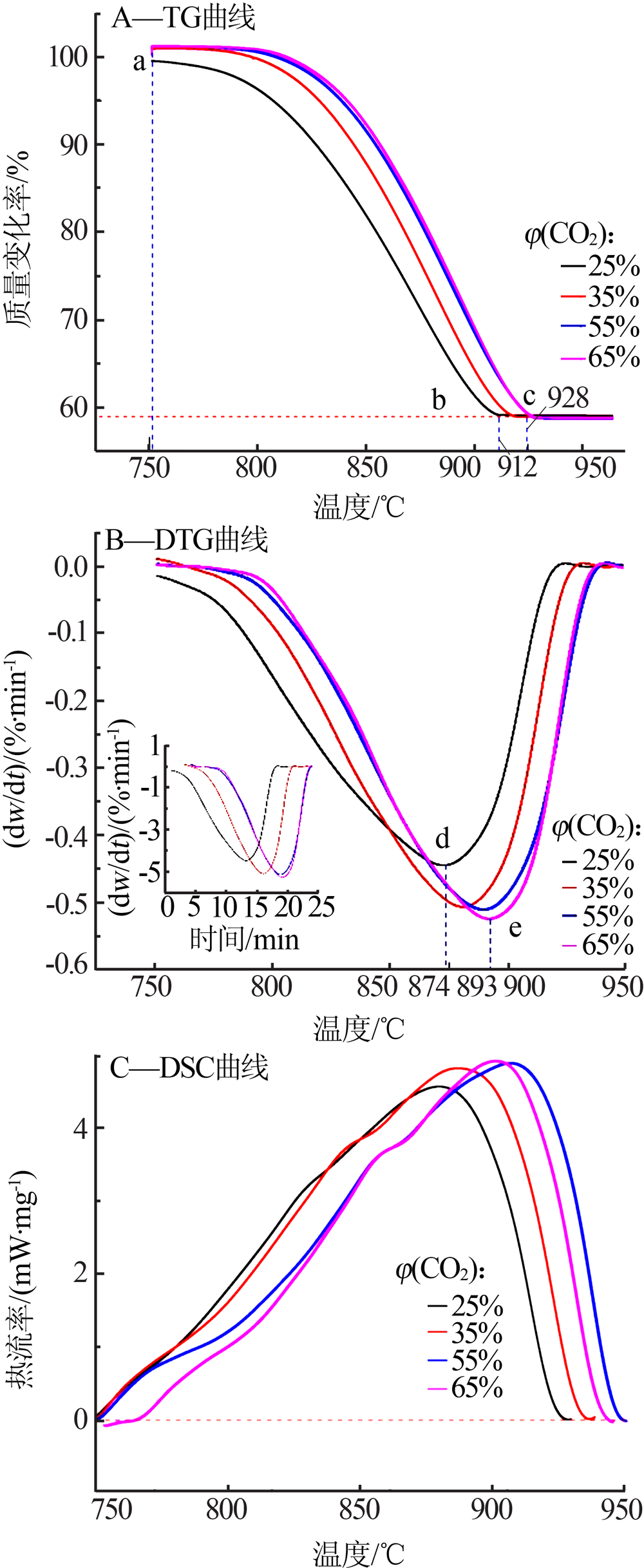
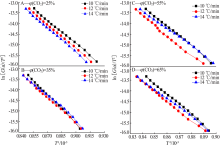
Inorganic Chemicals Industry ›› 2020, Vol. 52 ›› Issue (3): 59-63.doi: 10.11962/1006-4990.2019-0209
• Research & Development • Previous Articles Next Articles
Effect on kinetics of limestone decomposition under different CO2 atmospheres
Zhang Wenxian1,Liu Liansheng1( ),Cao Hejun2,Wu Binke2,Cheng Zhenpeng2
),Cao Hejun2,Wu Binke2,Cheng Zhenpeng2
- 1. School of Energy and Environmental Engineering,Hebei University of Technology,Tianjin 300401,China
2. Shijiazhuang Shenghongda Thermal Energy Engineering Technology Company Ltd.
-
Received:2019-09-28Online:2020-03-10Published:2020-03-31 -
Contact:Liu Liansheng E-mail:lane812@163.com
CLC Number:
Cite this article
Zhang Wenxian,Liu Liansheng,Cao Hejun,Wu Binke,Cheng Zhenpeng. Effect on kinetics of limestone decomposition under different CO2 atmospheres[J]. Inorganic Chemicals Industry, 2020, 52(3): 59-63.
share this article
"
| φ(CO2)/ % | n | E/(kJ·mol-1) | A/min-1 | R2 | E/(kJ·mol-1) | A/min-1 | R2 | E/(kJ·mol-1) | A/min-1 | R2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 10 ℃/min | 12 ℃/min | 14 ℃/min | ||||||||
| 25 | 1 | 346.976 3 | 7.74×1010 | 0.997 36 | 320.632 2 | 1.15×1011 | 0.997 86 | 304.310 4 | 1.46×1011 | 0.997 28 |
| 2/3 | 203.724 6 | 4.02×105 | 0.997 57 | 207.455 6 | 5.24×105 | 0.997 74 | 209.878 5 | 6.16×105 | 0.997 12 | |
| 2/1 | 148.098 7 | 8.29×102 | 0.997 43 | 150.867 2 | 1.01×103 | 0.997 61 | 152.662 6 | 1.14×103 | 0.996 94 | |
| 35 | 1 | 379.077 3 | 5.16×1013 | 0.998 02 | 348.221 0 | 6.72×1011 | 0.997 53 | 329.216 7 | 7.54×1011 | 0.997 32 |
| 2/3 | 246.379 3 | 3.26×107 | 0.997 91 | 219.128 4 | 1.83×106 | 0.997 40 | 219.759 8 | 2.08×106 | 0.997 15 | |
| 2/1 | 180.030 3 | 2.34×104 | 0.997 80 | 159.582 0 | 2.74×103 | 0.997 26 | 160.031 4 | 3.13×103 | 0.996 97 | |
| 55 | 1 | 397.806 1 | 9.22×1013 | 0.998 29 | 376.714 7 | 9.68×1013 | 0.998 29 | 355.665 7 | 1.06×1012 | 0.997 63 |
| 2/3 | 252.143 8 | 4.80×107 | 0.998 19 | 251.052 3 | 5.03×107 | 0.998 00 | 222.703 0 | 2.61×106 | 0.997 48 | |
| 2/1 | 184.312 6 | 3.14×104 | 0.998 09 | 183.221 2 | 3.29×104 | 0.998 10 | 162.221 6 | 3.71×103 | 0.997 32 | |
| 65 | 1 | 401.554 5 | 3.86×1014 | 0.997 83 | 382.227 8 | 4.86×1013 | 0.997 42 | 361.017 9 | 3.80×1012 | 0.997 64 |
| 2/3 | 261.305 5 | 1.26×108 | 0.997 72 | 248.392 7 | 3.30×107 | 0.997 29 | 231.580 9 | 6.20×106 | 0.997 51 | |
| 2/1 | 191.181 0 | 6.55×104 | 0.997 60 | 181.475 2 | 2.47×104 | 0.997 15 | 168.862 4 | 7.17×103 | 0.997 30 | |
| [1] | 郭汉杰, 尹志明, 王宏伟 . 冶金活性石灰烧制过程最佳工艺制度[J]. 北京科技大学学报, 2008,30(2):148-151. |
| [2] | 李宏, 曲英 . 氧气转炉炼钢用石灰石代替石灰节能减排初探[J]. 中国冶金, 2010,20(9):45-48. |
| [3] | 汤明伟 . 一种石灰窑尾气分离提纯工艺技术研究及应用[J]. 中国氯碱, 2017(5):42-44. |
| [4] | 杨磊, 许兴存 . 燃煤电厂锅炉尾气排放脱除CO2的流程模拟及研究[J]. 资源节约与环保, 2014(9):133-134. |
| [5] | 黄希祜 . 钢铁冶金原理[M]. 3版.北京: 冶金工业出版社, 2002. |
| [6] | 陈凯锋, 薛正良, 李建立 . 高温煅烧下快速加热石灰石的热分解反应动力学[J]. 硅酸盐学报, 2016,44(5):754-762. |
| [7] | 胡彬, 薛正良, 白莎 , 等. 石灰石高温快速煅烧分解反应动力学研究[J]. 炼钢, 2017,33(1):56-61. |
| [8] | 王世杰 . 水泥预分解窑系统内生料分解、煤粉燃烧与NOx控制研究[D]. 武汉:华中科技大学, 2006. |
| [9] | 范浩杰, 章明川, 吴国新 , 等. 碳酸钙热分解的机理研究[J]. 动力工程, 1998,18(5):40-43. |
| [10] | Criado J M, González M, Málek J , et al. The effect of the CO2 pres-sure on the thermal decomposition kinetics of calcium carbona-te[J]. Thermochimical Acta, 1995,254:121-127. |
| [11] | Wei H, Luo Y . A study on the kinetics of thermal decomposition of CaCO3[J]. Journal of Thermal Analysis, 1995,45(1/2):303-310. |
| [12] | 郑瑛, 陈小华, 周英彪 , 等. CaCO3分解机理和动力学参数的研究[J]. 华中科技大学学报:自然科学版, 2002,30(12):86-88. |
| [13] | 郑瑛, 宋侃, 池保华 , 等. 二氧化碳气氛下碳酸钙热分解动力学研究[J]. 华中科技大学学报:自然科学版, 2007,35(8):87-89. |
| [14] | 曹静, 乔秀臣, 柳成亮 , 等. 石灰石在二氧化碳与空气混合气氛下的分解动力学[J]. 无机盐工业, 2016,48(12):32-36. |
| [15] | 李辉, 张乐乐, 段永华 , 等. 高二氧化碳浓度下石灰石的热分解反应动力学[J]. 硅酸盐学报, 2013,41(5):637-643. |
| [16] | Ávila I, Crnkovic P M, Milioli F E , et al. Thermal decomposition kinetics of Brazilian limestones:effect of CO2 partial pressure[J]. Environmental Technology, 2012,33(10):1175-1182. |
| [17] | 郑瑛, 陈小华, 郑楚光 . CaCO3分解机理的研究[J]. 动力工程, 2004,24(2):280-284. |
| [18] | 日本化学会. 无机固态反应[M]. 董万堂, 董绍俊译.北京:科学出版社, 1985. |
| [19] | 冯云, 陈延信 . 碳酸钙的分解动力学研究进展[J]. 硅酸盐通报, 2006,25(3):140-145,154. |
| [20] | 胡荣祖, 史启祯 . 热分析动力学[M]. 2版.北京: 科学出版社, 2008. |
| [1] | Yan Xin,Wu Jianyi,Lu Yunfeng,Yan Ziteng. Study on new technology of comprehensive utilization of alkali residue in ammonia alkali plant [J]. Inorganic Chemicals Industry, 2021, 53(1): 68-71. |
| [2] | Zhang Bin,Xiao Xiao,Han Yunjiao,Zhang Yuanyuan,Yu Hongwei. Study on third-step infrared spectroscopy of calcium carbonate [J]. Inorganic Chemicals Industry, 2021, 53(1): 97-101. |
| [3] | Yan Xin. Research and production of colorful nano-sized calcium carbonate [J]. Inorganic Chemicals Industry, 2020, 52(5): 53-55. |
| [4] | Jing Ting. Study on extrudability of silicone sealant by using modified nano-sized calcium carbonate [J]. Inorganic Chemicals Industry, 2020, 52(4): 57-60. |
| [5] | Wang Qianqian,Bai Chunhua,Ren Hui,Li Guanghui. Crystal structure regulation of calcium carbonate by adding tetradecanoic acid [J]. Inorganic Chemicals Industry, 2020, 52(4): 29-32. |
| [6] | Wang Yubin,Wang Wangbo,Zhu Xinfeng,Xun Jingwen,Dang Weiben. Effect of different magnesium salts on solubility behavior of calcium carbonate under magnetization [J]. Inorganic Chemicals Industry, 2020, 52(3): 35-38. |
| [7] | Wang Gang,Tang Shengwei,Chen Yanxiao,Zhang Tao. Effect of CTAB on crystal form of CaCO3 in indirect mineralization of CO2 by CaSO4·2H2O [J]. Inorganic Chemicals Industry, 2020, 52(3): 75-79. |
| [8] | Chang Yuefan,Zhang Huijie,Wang Shanshan,Xue Yongqiang. Controllable preparation of nano-calcium carbonate with different particle sizes [J]. Inorganic Chemicals Industry, 2020, 52(12): 29-33. |
| [9] | Liu Baoshu,Hao Zhigang,Ma Yongshan,Liu Runjing,Hu Yongqi. Trend analysis and review of calcium carbonate production in China for the past 60 years [J]. Inorganic Chemicals Industry, 2020, 52(10): 37-43. |
| [10] | Liu Zaitong,Li Chang′e,Yang Yuling,Li Guoting. Preparation of sub-micron spindle-shaped calcium carbonate and sodium hydroxide co-production by causticizing method [J]. Inorganic Chemicals Industry, 2019, 51(8): 56-59. |
| [11] | Liu Jianlu,Liu Xia,Yue Maowen. Study on scaling tendency in nanofiltration process by treating underground brine in Laizhou bay,Bohai [J]. Inorganic Chemicals Industry, 2019, 51(8): 64-68. |
| [12] | Yan Xin1,Lu Yunfeng2,Ma Yuanyuan3,Xie Long3. Study on composite carbonization mechanism of nano calcium carbonateproduced by calcium chloride-ammonia water system [J]. Inorganic Chemicals Industry, 2019, 51(7): 77-80. |
| [13] | Zhang Pinghua,Yan Yunjie,Chen Jianjun,Wang Hongyan,Zhang Chunli,Wu Ning. Preparation of nanometer calcium carbonate from high-Ca-Mg phosphorus tailings by acid leaching [J]. Inorganic Chemicals Industry, 2019, 51(3): 63-66. |
| [14] | Tan Tingting1,2,3,Zhong Jianchu1,2,3. Study on controllable synthesis of spherical calcium carbonate [J]. Inorganic Chemicals Industry, 2019, 51(12): 30-34. |
| [15] | CHEN Bo, SONG Xing-Fu, XU Yan-Xia, SUN Yu-Zhu, YU Jian-Guo. Optimization of preparation of calcium carbonate from continuous crystallization process of ammonium carbonate and conversion of calcium sulfate by response surface methodology [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(9): 18-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|