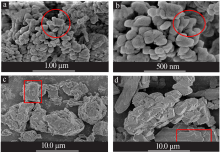
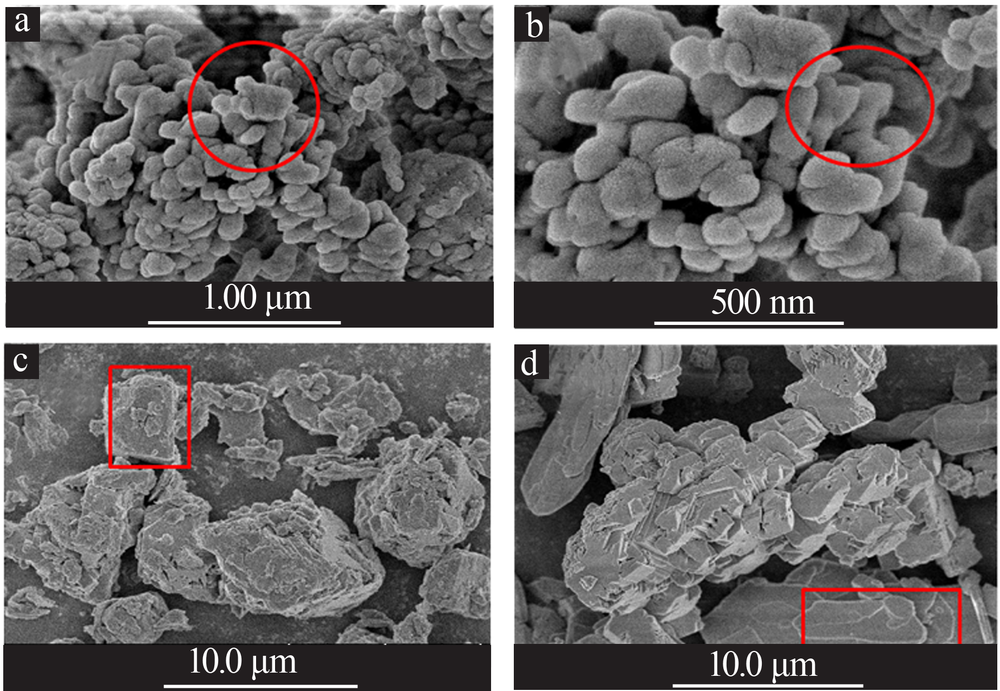
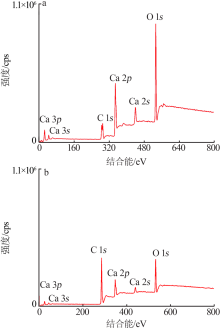
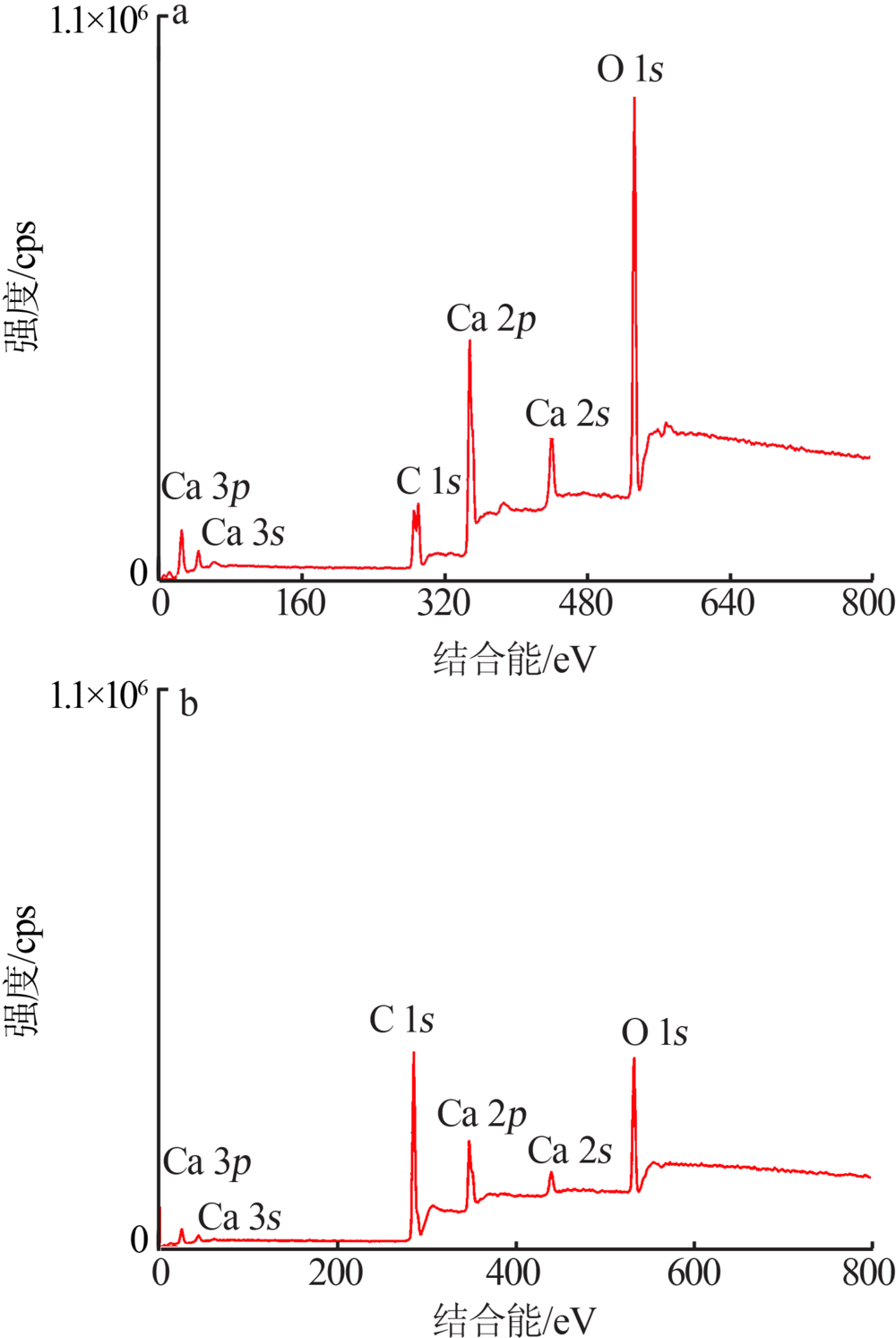
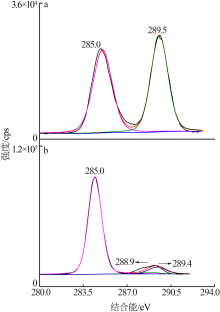
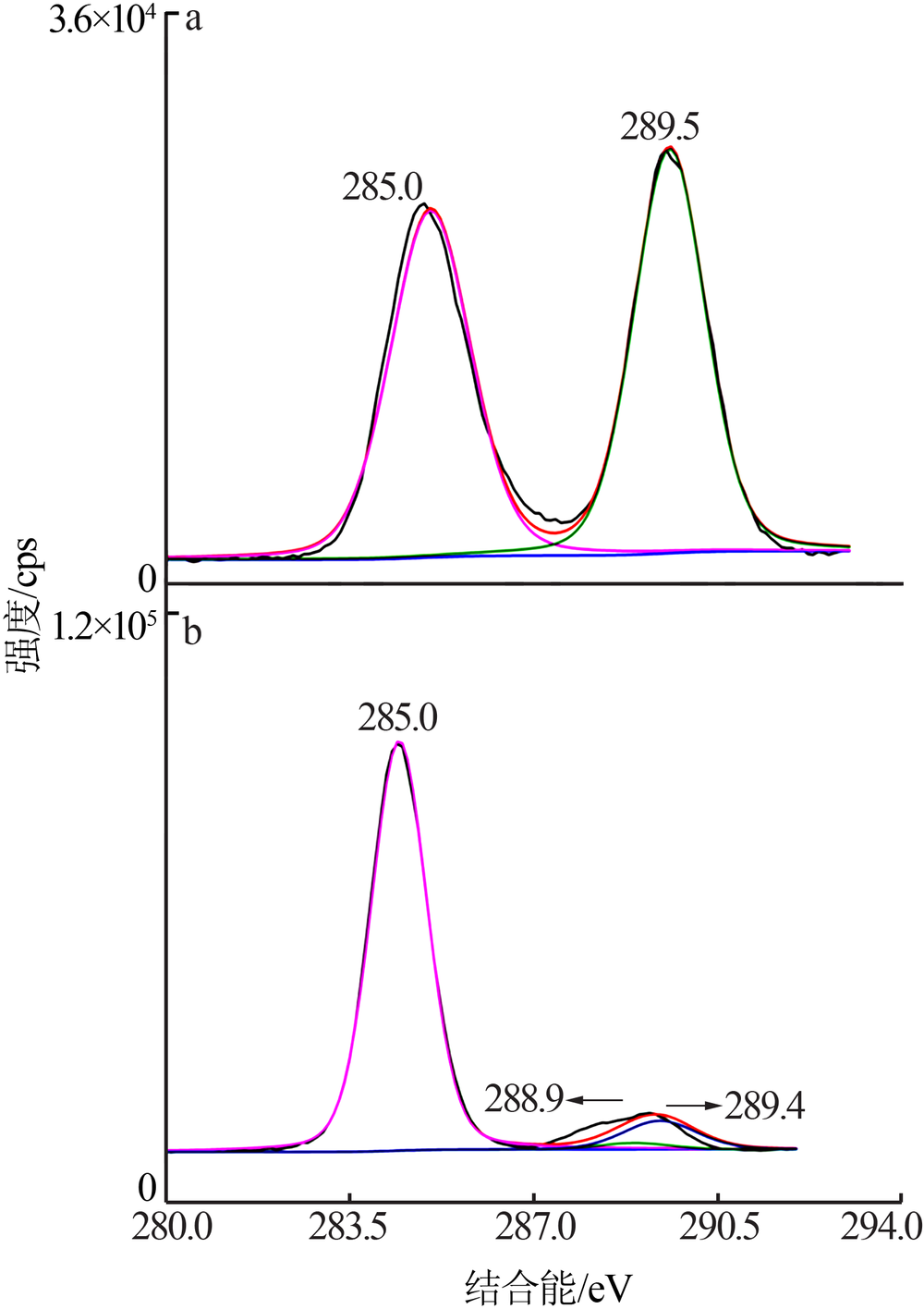
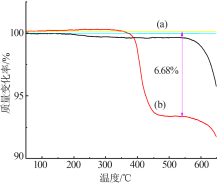
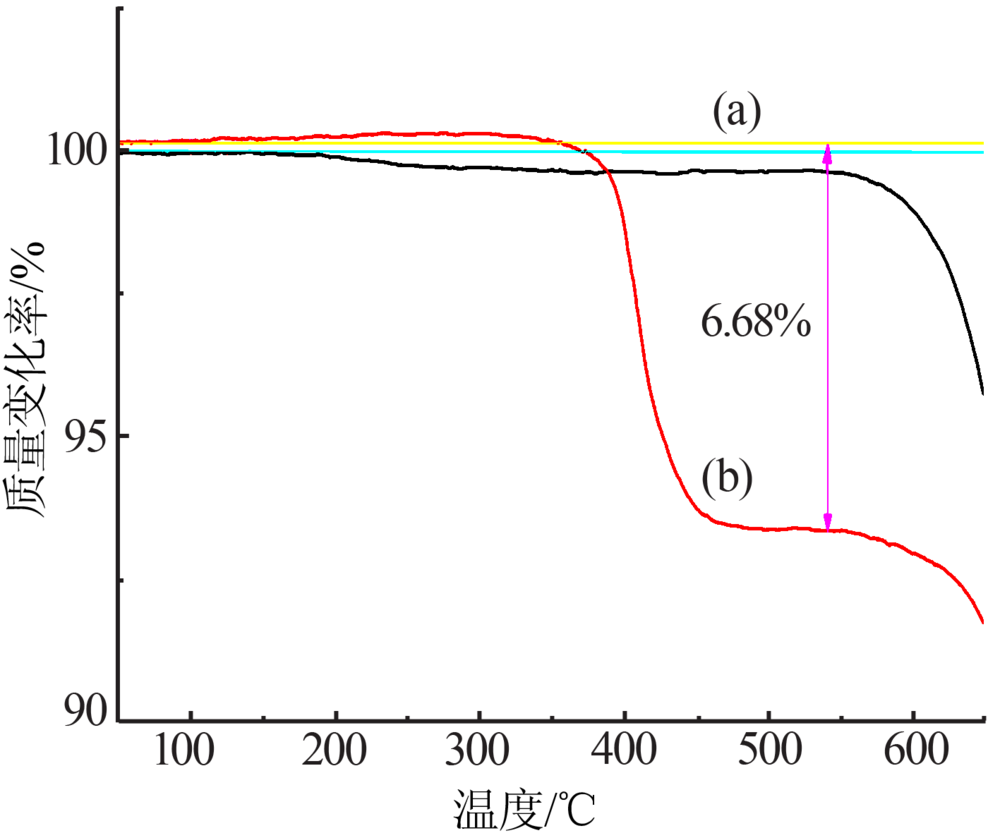
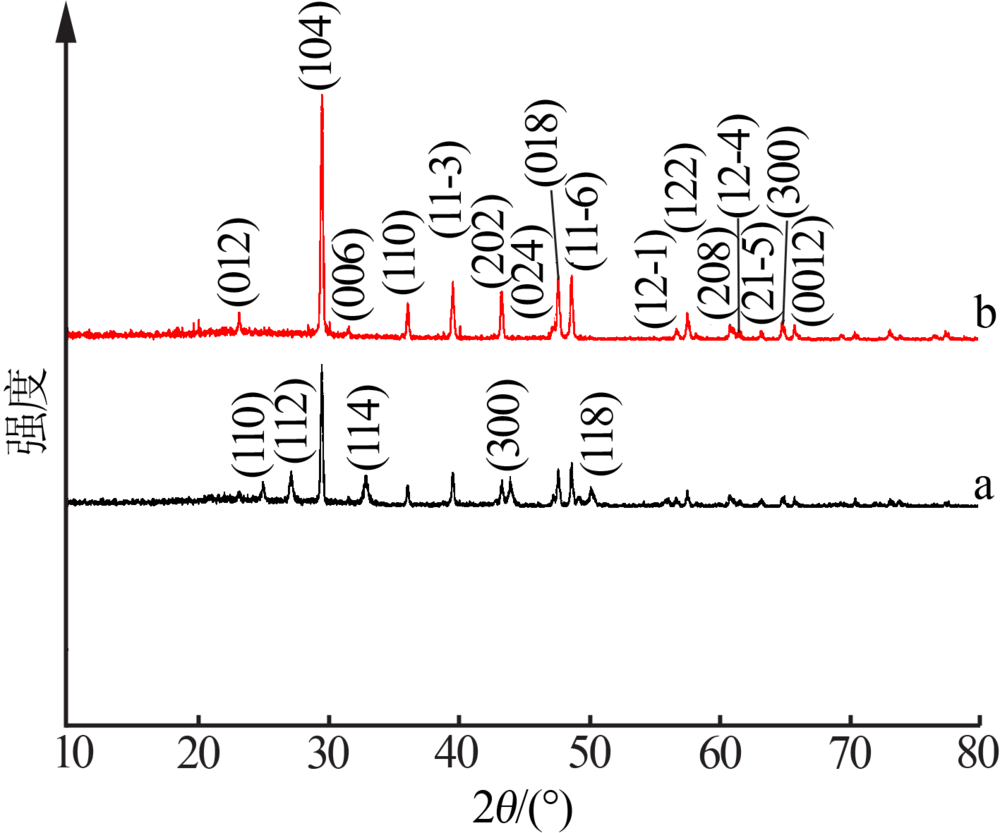
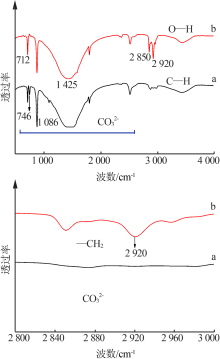
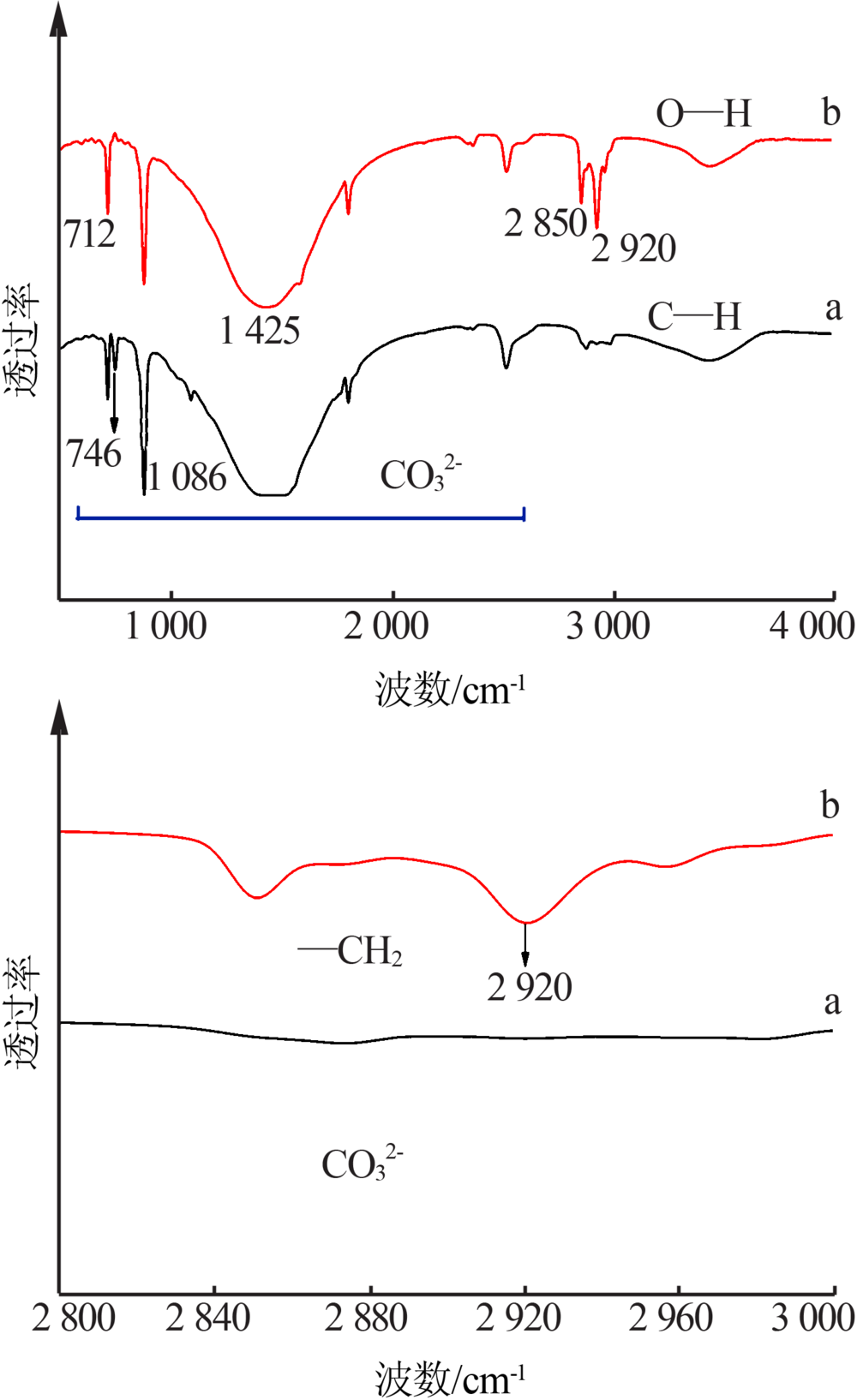
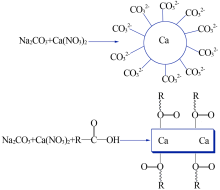
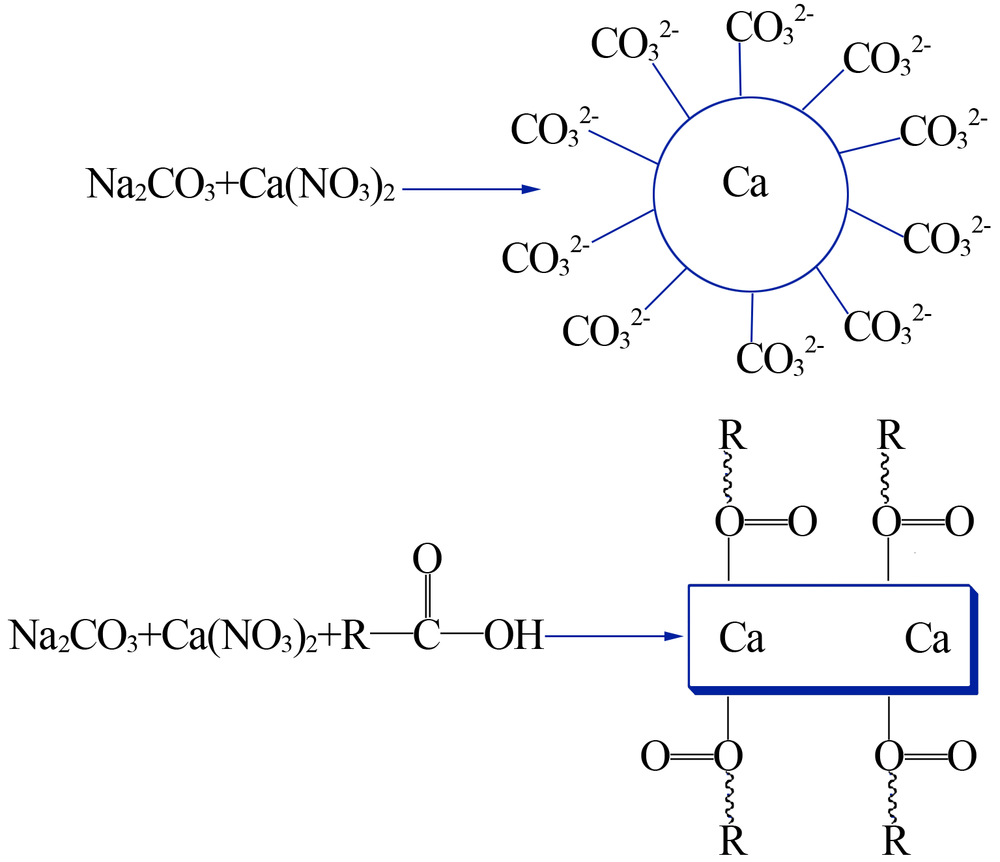
| [1] |
陆东东, 曹石林, 马晓娟 , 等. 淀粉包覆型碳酸钙填料的制备及其在造纸中的应用[J]. 造纸科学与技术, 2015,34(3):42-48.
|
| [2] |
于洋 . 造纸用碳酸钙粒子的合成及表征[D]. 长春:吉林大学, 2008.
|
| [3] |
徐永华, 邹勇, 傅俊祥 , 等. 不同重质碳酸钙在聚酯粉末涂料中的应用对比研究[J]. 涂料技术与文摘, 2017,38(5):41-44.
|
| [4] |
骆振福, 任晓玲, 乔军 , 等. 不同品种碳酸钙填充PVC性能的研究[J]. 中国矿业大学学报, 2012,41(1):69-73.
|
| [5] |
张亨 . 碳酸钙的生产及其在塑料中的应用研究[J].橡塑资源利用, 2010(3):16-20.
|
| [6] |
赵丽娜 . 碳酸钙的形貌控制及表面改性研究[D]. 吉林:吉林大学, 2009.
|
| [7] |
王友, 曾一文, 覃康玉 , 等. 硬脂酸-钛酸酯偶联剂改性重质碳酸钙粉体研究[J]. 无机盐工业, 2016,48(6):38-40.
|
| [8] |
郝丽丽 . 纳米碳酸钙的表面改性及对Cu 2+吸附性能的研究 [D]. 西安:西安电子科技大学, 2017.
|
| [9] |
赖俊伟 . 改性脂肪酸对重质碳酸钙的表面修饰及其在聚合物中的应用[D]. 广州:广东工业大学, 2012.
|
| [10] |
周国永, 曾一文, 黄志强 , 等. P(BA-FA)原位聚合法改性重质碳酸钙微粒的研究[J]. 无机盐工业, 2013,45(4):18-21.
|
| [11] |
王明, 丁杨, 闫红旭 , 等. 碳化法形貌可控制备碳酸钙的研究[J]. 无机盐工业, 2018,50(12):37-40.
|
| [12] |
宋丽英, 胡晓湘, 胡庆福 , 等. 中国纳米级碳酸钙生产现状与发展建议[J]. 无机盐工业, 2010,42(6):1-4.
|
| [13] |
陈银霞, 纪献兵 . 复分解法原位合成疏水球状球霰石纳米碳酸钙[J]. 无机盐工业, 2014,46(4):25-28.
|
| [14] |
王兴权 . 碳化法、复分解法和微乳法制备不同形貌超细碳酸钙[D]. 兰州:兰州交通大学, 2013.
|
| [15] |
杨立静, 杨凤东, 陈银霞 , 等. 油酸对复分解法制备纳米碳酸钙的影响[J]. 材料导报B:研究篇, 2013,27(11):55-58.
|
| [16] |
刘立华 . 硬脂酸钙改性纳米碳酸钙的效果研究[J]. 化工科技市场, 2010,33(10):14-16.
|
| [17] |
Hu Zeshan, Deng Yulin . Superhy drophobic surface fabricated from fatty acid-modified precipitated calcium carbonate[J]. J.Ind.Eng.Chem.Res., 2010,49:5625-5630.
|
| [18] |
刘杰 . 对微纳米CaCO3的形貌、尺度和均匀度的控制研究[D]. 武汉:湖北工业大学, 2012.
|
 ),Ren Hui2,Li Guanghui2
),Ren Hui2,Li Guanghui2