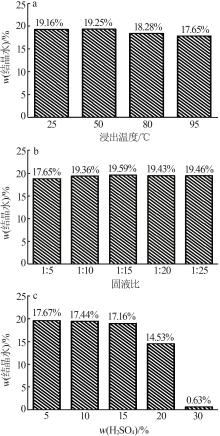
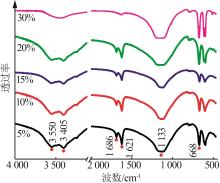
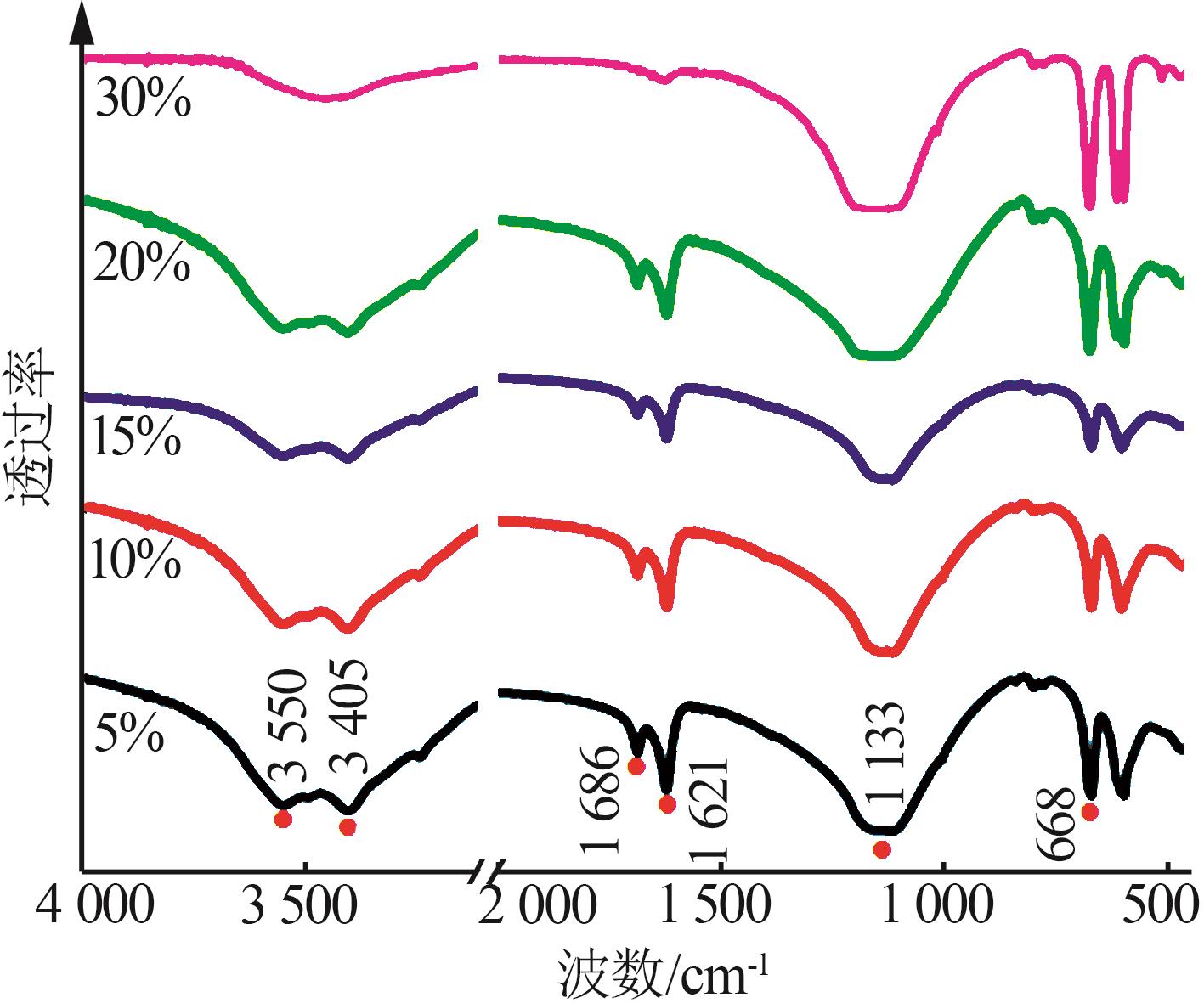
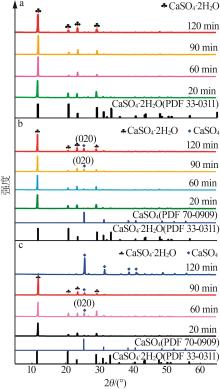
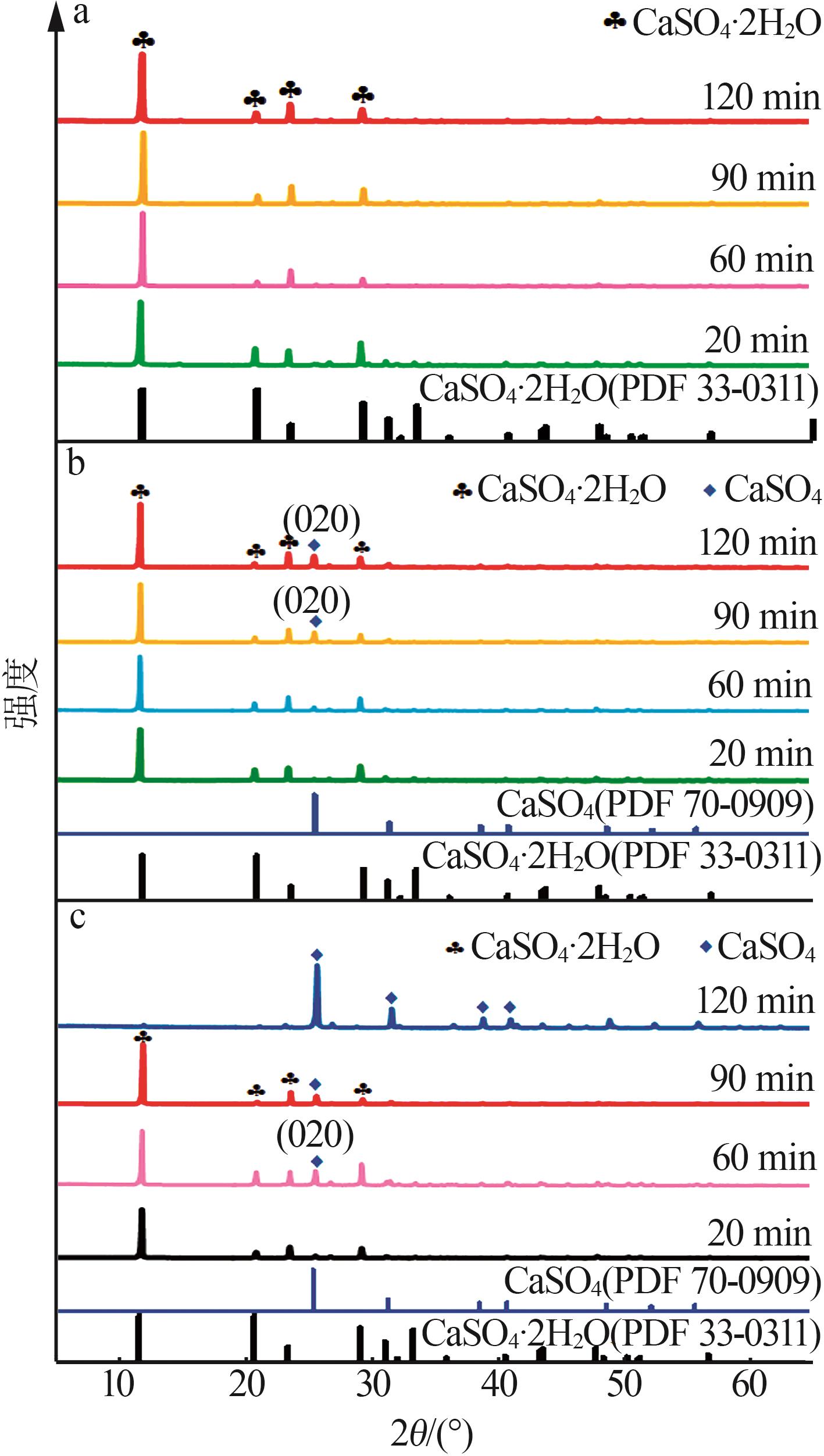
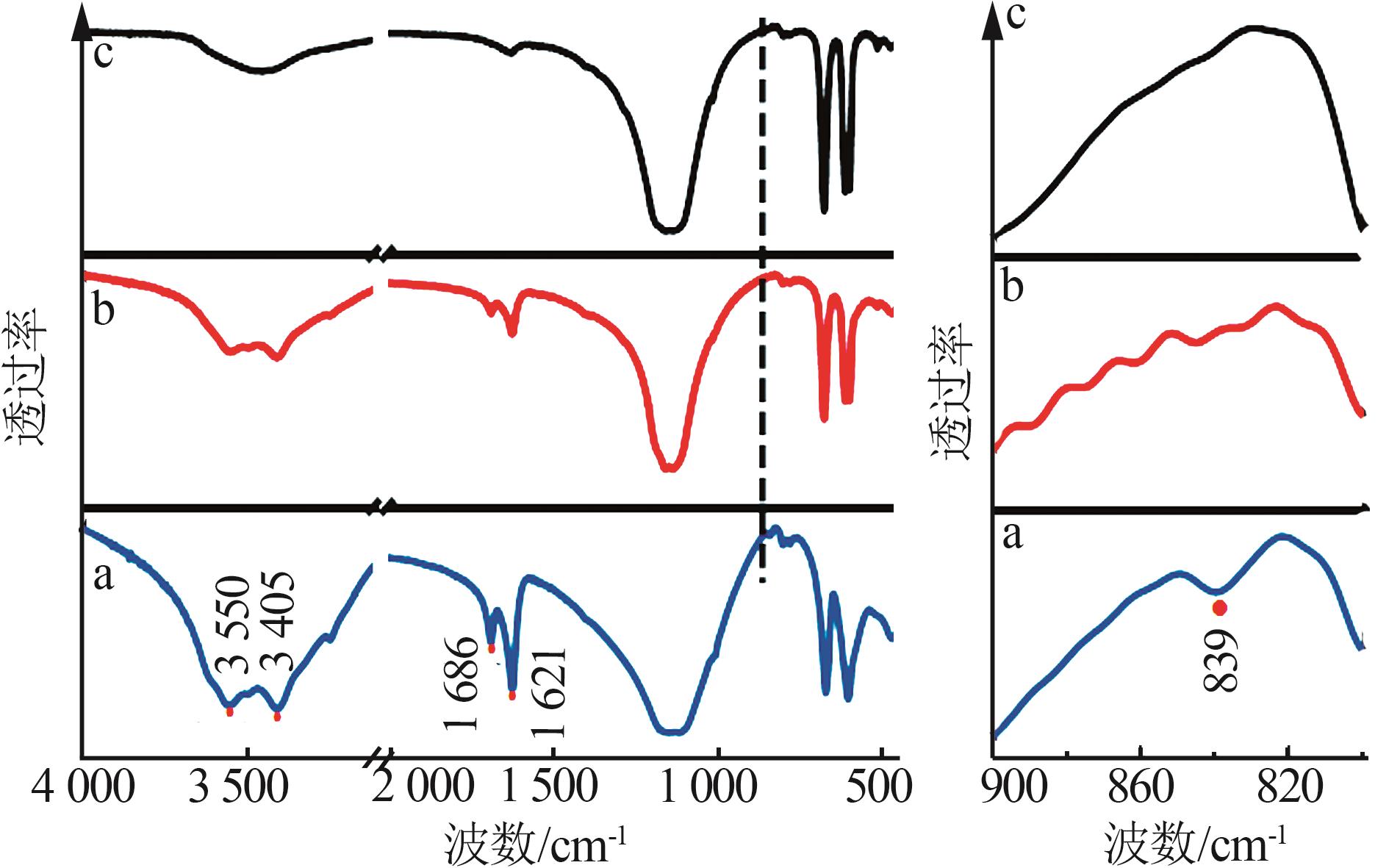
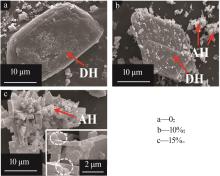
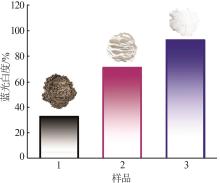
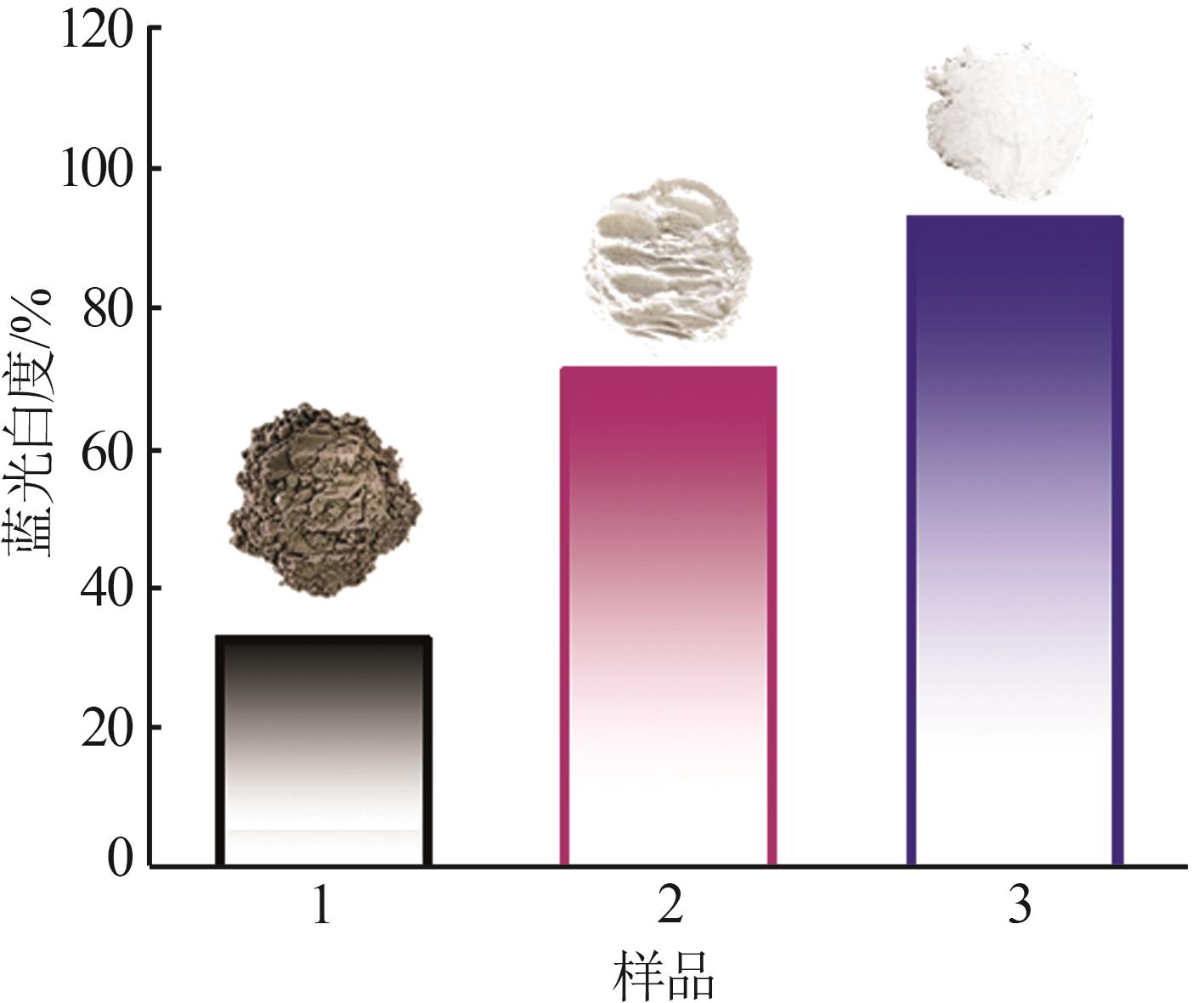
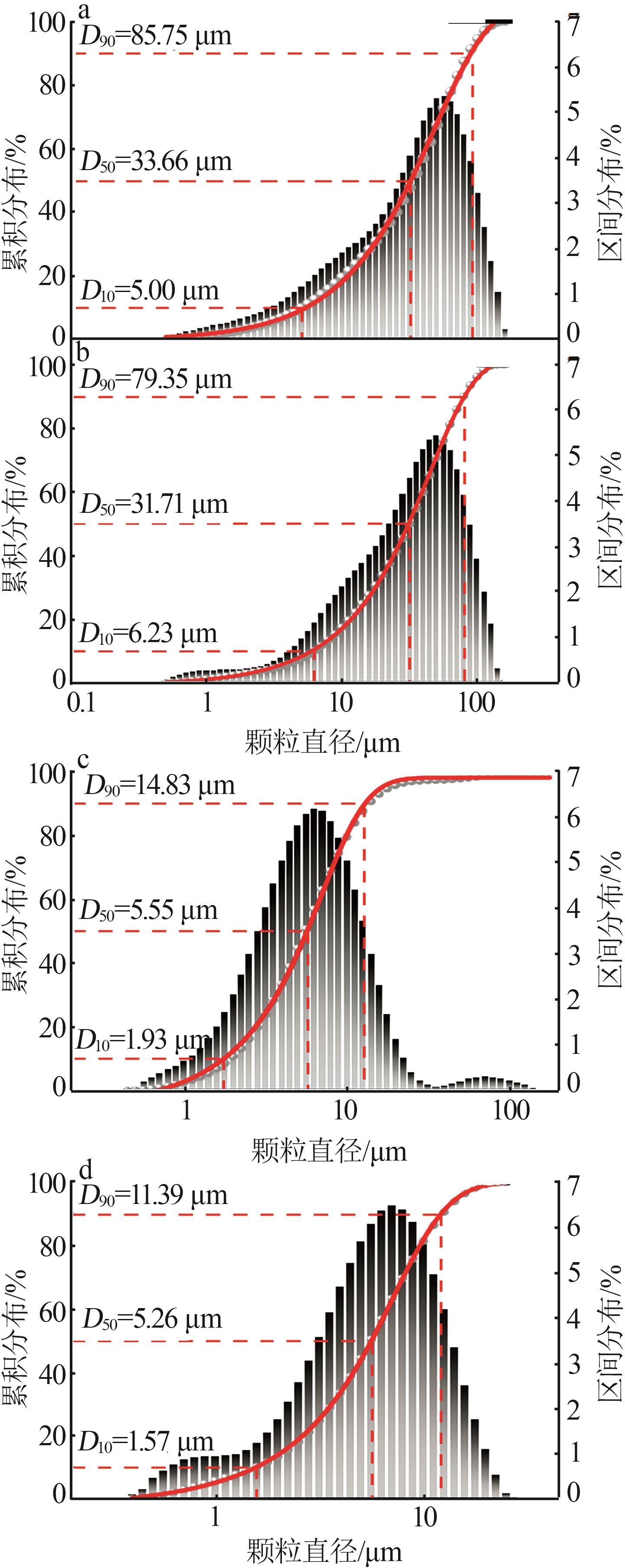
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (5): 101-107.doi: 10.19964/j.issn.1006-4990.2023-0301
• Environment·Health·Safety • Previous Articles Next Articles
Study on effect of crystallizer on preparation of anhydrous calcium sulfate from phosphogypsum
WANG Mingshun( ), AO Xianquan(
), AO Xianquan( ), YUAN Xing, DONG Wenyan, CHEN Qianlin
), YUAN Xing, DONG Wenyan, CHEN Qianlin
- College of Chemistry and Chemical Engineering,Guizhou University,Guiyang 550025,China
-
Received:2023-06-01Online:2024-05-10Published:2024-05-15 -
Contact:AO Xianquan E-mail:1349490778@qq.com;aoxianquan@163.com
CLC Number:
Cite this article
WANG Mingshun, AO Xianquan, YUAN Xing, DONG Wenyan, CHEN Qianlin. Study on effect of crystallizer on preparation of anhydrous calcium sulfate from phosphogypsum[J]. Inorganic Chemicals Industry, 2024, 56(5): 101-107.
share this article
| 1 | CHEN Shun, CHEN Jizhan, HE Xingyang,et al.Micromicelle-mechanical coupling method for high-efficiency phosphorus removal and whiteness improvement of phosphogypsum[J].Construction and Building Materials,2022,354:129220. |
| 2 | FANG Ji, GE Yingyong, CHEN Zhijie,et al.Flotation purification of waste high-silica phosphogypsum[J].Journal of Environmental Management,2022,320:115824. |
| 3 | 吴宝建,吕昕峰,张清杰,等.磷石膏湿法偶联除杂规律初探[J].无机盐工业,2020,52(12):55-58. |
| WU Baojian, Xinfeng LÜ, ZHANG Qingjie,et al.Primary exploration of removing impurities from phosphogypsum by wet coupling process[J].Inorganic Chemicals Industry,2020,52(12):55-58. | |
| 4 | CHEN Xuemei, GAO Jianming, ZHAO Yasong.Investigation on the hydration of hemihydrate phosphogypsum after post treatment[J].Construction and Building Materials,2019,229:116864. |
| 5 | 顾青山,林喜华,赵士豪,等.不同预处理工艺对磷石膏性能的影响[J].无机盐工业,2022,54(4):17-23. |
| GU Qingshan, LIN Xihua, ZHAO Shihao,et al.Effect of different pretreatment processes on properties of phosphogypsum[J].Inorganic Chemicals Industry,2022,54(4):17-23. | |
| 6 | ROSALES J, PÉREZ S M, CABRERA M,et al.Treated phosphogypsum as an alternative set regulator and mineral addition in cement production[J].Journal of Cleaner Production,2020,244:118752. |
| 7 | RASHAD A M.Phosphogypsum as a construction material[J].Journal of Cleaner Production,2017,166:732-743. |
| 8 | 王进明,董发勤,王肇嘉,等.磷石膏浮选增白净化新工艺研究[J].非金属矿,2019,42(5):1-5. |
| WANG Jinming, DONG Faqin, WANG Zhaojia,et al.Study on new technology of phosphogypsum whitening and purification by flotation[J].Non-Metallic Mines,2019,42(5):1-5. | |
| 9 | 方官涛,敖先权,刘境,等.磷石膏煅烧过程中添加剂对其杂质和白度影响[J].非金属矿,2019,42(4):10-12. |
| FANG Guantao, AO Xianquan, LIU Jing,et al.Effects of additives on impurities and whiteness of phosphogypsum during calcining[J].Non-Metallic Mines,2019,42(4):10-12. | |
| 10 | LIU Yikai, ZHANG Qinli, CHEN Qiusong,et al.Utilisation of water-washing pre-treated phosphogypsum for cemented paste backfill[J].Minerals,2019,9(3):175. |
| 11 | GONG Shang, LI Xiaolong, SONG Fangxiang,et al.Preparation and application in HDPE of nano-CaSO4 from phosphogypsum[J].ACS Sustainable Chemistry & Engineering,2020,8(11):4511-4520. |
| 12 | LIU Hao, NIE Chenchen, LI Hongping,et al.Hydrophobically modified phosphogypsum and its application in polypropylene composites[J].Construction and Building Materials,2022,347:128500. |
| 13 | 田家新,彭伟军,苗毅恒,等.磷石膏漂白—煅烧增白工艺研究[J].矿产保护与利用,2021,41(3):76-80. |
| TIAN Jiaxin, PENG Weijun, MIAO Yiheng,et al.Study on whitening of phosphogypsum via bleaching and calcination[J].Conservation and Utilization of Mineral Resources,2021,41(3):76-80. | |
| 14 | XU Li, FANG Keneng, BI Yongxiang,et al.Preparation of anhydrous micron CaSO4 with different morphologies from phosphogypsum and its reinforcing in polyvinyl chloride[J].Construction and Building Materials,2023,365:130126. |
| 15 | GUAN Qingjun, SUI Ying, YU Weijian,et al.Deep removal of phosphorus and synchronous preparation of high-strength gypsum from phosphogypsum by crystal modification in NaCl-HCl solutions[J].Separation and Purification Technology,2022,298:121592. |
| 16 | WALAWALKAR M, NICHOL C K, AZIMI G.Process investigation of the acid leaching of rare earth elements from phosphogypsum using HCl,HNO3,and H2SO4 [J].Hydrometallurgy,2016,166:195-204. |
| 17 | QI Mengyao, PENG Weijun, WANG Wen,et al.Simple and efficient method for purification and recovery of gypsum from phosphogypsum:Reverse-direct flotation and mechanism[J].Journal of Molecular Liquids,2023,371.Doi:10.1016/j.molliq.2022.121111. |
| 18 | GUPTA K, SINGH S, RAMACHANDRA RAO M S.Direct and facile room-temperature synthesis of nanocrystalline calcium sulfate dihydrate(gypsum)[J].Crystal Growth & Design,2016, 16(6):3256-3261. |
| 19 | MA Baoguo, LU Wenda, SU Ying,et al.Synthesis of α-hemihydrate gypsum from cleaner phosphogypsum[J].Journal of Cleaner Production,2018,195:396-405. |
| 20 | LI Xianbo, ZHANG Qin, SHEN Zhihui,et al.L-aspartic acid:A crystal modifier for preparation of hemihydrate from phosphogypsum in CaCl2 solution[J].Journal of Crystal Growth,2019,511:48-55. |
| 21 | YU Bingjie, MIAO Shanshan, DING Manxin,et al.Solubility and physical properties of α-calcium sulfate hemihydrate in NaCl and glycerol aqueous solutions at 303.15,323.15,and 343.15 K[J].Journal of Chemical & Engineering Data,2021,66(10):3686-3694. |
| 22 | JIANG Guangming, FU Hailu, SAVINO K,et al.Nonlattice cation-SO42- ion pairs in calcium sulfate hemihydrate nucleation[J].Crystal Growth & Design,2013,13(11):5128-5134. |
| 23 | 汪潇,曹博伦,金彪,等.添加剂调控半水石膏晶体生长研究进展[J].硅酸盐学报,2020,48(1):94-103. |
| WANG Xiao, CAO Bolun, JIN Biao,et al.Using additives to control crystal growth of hemihydrate gypsum:A short review[J].Journal of the Chinese Ceramic Society,2020,48(1):94-103. |
| [1] | LENG Manxi, ZHU Yu, YAN Jikang, XIE Ke, ZOU Yongjie. Study on crystallization characteristics and mineral flotation performance of industrial waste phosphogypsum [J]. Inorganic Chemicals Industry, 2025, 57(3): 108-115. |
| [2] | TAN Shanyi, WEN Huizi, HE Shuyu, ZHANG Liwen, CHEN Shaohua, XI Benjun. Study on leaching behavior and kinetics of phosphorus from phosphogypsum [J]. Inorganic Chemicals Industry, 2025, 57(2): 105-112. |
| [3] | TANG Kaijing, LIU Chuanbei, LI Yingding, JIANG Yong, WU Junnan, ZHANG Tao. Research on preparation and mechanism of superhydrophobic phosphogypsum products [J]. Inorganic Chemicals Industry, 2025, 57(1): 97-102. |
| [4] | TANG Xuemei, WANG Meibo, XU Li, ZHANG Xujie, TAI Shijun, YI Xianmei, LIU Hongjuan, PAN Linyi. Study on whitening of phosphogypsum by low⁃temperature calcination [J]. Inorganic Chemicals Industry, 2024, 56(8): 110-115. |
| [5] | SUN Lan, CHEN Shiying, YANG Liuxu, NIU Yiming, ZHAO Aonan. Basic study on fixing soluble phosphorus in phosphogypsum with magnesium slag [J]. Inorganic Chemicals Industry, 2024, 56(8): 92-98. |
| [6] | HU Cheng, LIU Meng, XIANG Weiheng, CHEN Ping, WANG Neng, LU Guanju, ZHOU Jinlan. Preparation of α-hemihydrous gypsum from CaCl2 and MgCl2 and their composite solution [J]. Inorganic Chemicals Industry, 2024, 56(7): 112-117. |
| [7] | HU Dian, GUO Ze, ZHANG Hanquan, LU Manman. Research on effects of roasting process and typical impurities on reduction and decomposition process of phosphogypsum [J]. Inorganic Chemicals Industry, 2024, 56(7): 88-95. |
| [8] | HU Cheng, LIU Meng, XIANG Weiheng, DUAN Pengxuan, LI Shunkai, MING Yang, WANG Neng, LU Guanju. Effect of NaCl solution concentration on transcrystallization behavior of α-hemihydrate gypsum from phosphogypsum [J]. Inorganic Chemicals Industry, 2024, 56(6): 87-93. |
| [9] | TU Yanping, BAI Dengxian, CHENG Shukai, XIE Junjie, HUANG Zhiliang, CHEN Guofu. Effect of high temperature modification of mineral powder and quicklime on properties of phosphogypsum cement based materials [J]. Inorganic Chemicals Industry, 2024, 56(6): 94-101. |
| [10] | DENG Fuli, XIA Zhixiang, LONG Bingwen, ZHANG Yi, DAI Yafen, WANG Bin, DING Yigang. Study on purification process of phosphogypsum by reverse flotation [J]. Inorganic Chemicals Industry, 2024, 56(5): 115-120. |
| [11] | WANG Peixiong, GONG Xiaomei, DING Jiaqi, CAO Hong. Effect of crystal modifier on preparation of α-hemihydrates gypsum from industrial gypsum [J]. Inorganic Chemicals Industry, 2024, 56(4): 112-117. |
| [12] | CHEN Feng, FENG Kang, LI Ming, SHEN Haojie, TIAN Chengtao, TANG Yuan, LI Zhili, HE Dongsheng. Application of organically modified calcium sulfate whiskers in asphalt modification [J]. Inorganic Chemicals Industry, 2024, 56(3): 125-130. |
| [13] | WANG Ruting, ZHAO Xiaorong, HUANG Xuquan, WANG Haojie, XUE Fei, CAI Jiawei. Research on preparation and early performance of mixed phase phosphogypsum-based cementing materials [J]. Inorganic Chemicals Industry, 2024, 56(3): 98-104. |
| [14] | ZHANG Guidong, YANG Xuejiao, GUO Xudong, YANG Lin. Study on hydration properties of type Ⅱ anhydrite prepared by calcination and atmospheric acidification [J]. Inorganic Chemicals Industry, 2024, 56(2): 104-110. |
| [15] | WANG Yanyu, GU Shouyu, HOU Cuihong, JING Hongquan, GUAN Hongling, ZHANG Hui. Sulfur escape and slag physical phase analysis by carbon thermal reduction melting based on phosphogypsum ingredients [J]. Inorganic Chemicals Industry, 2024, 56(2): 86-94. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||