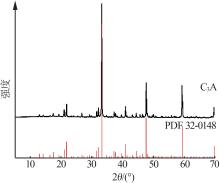
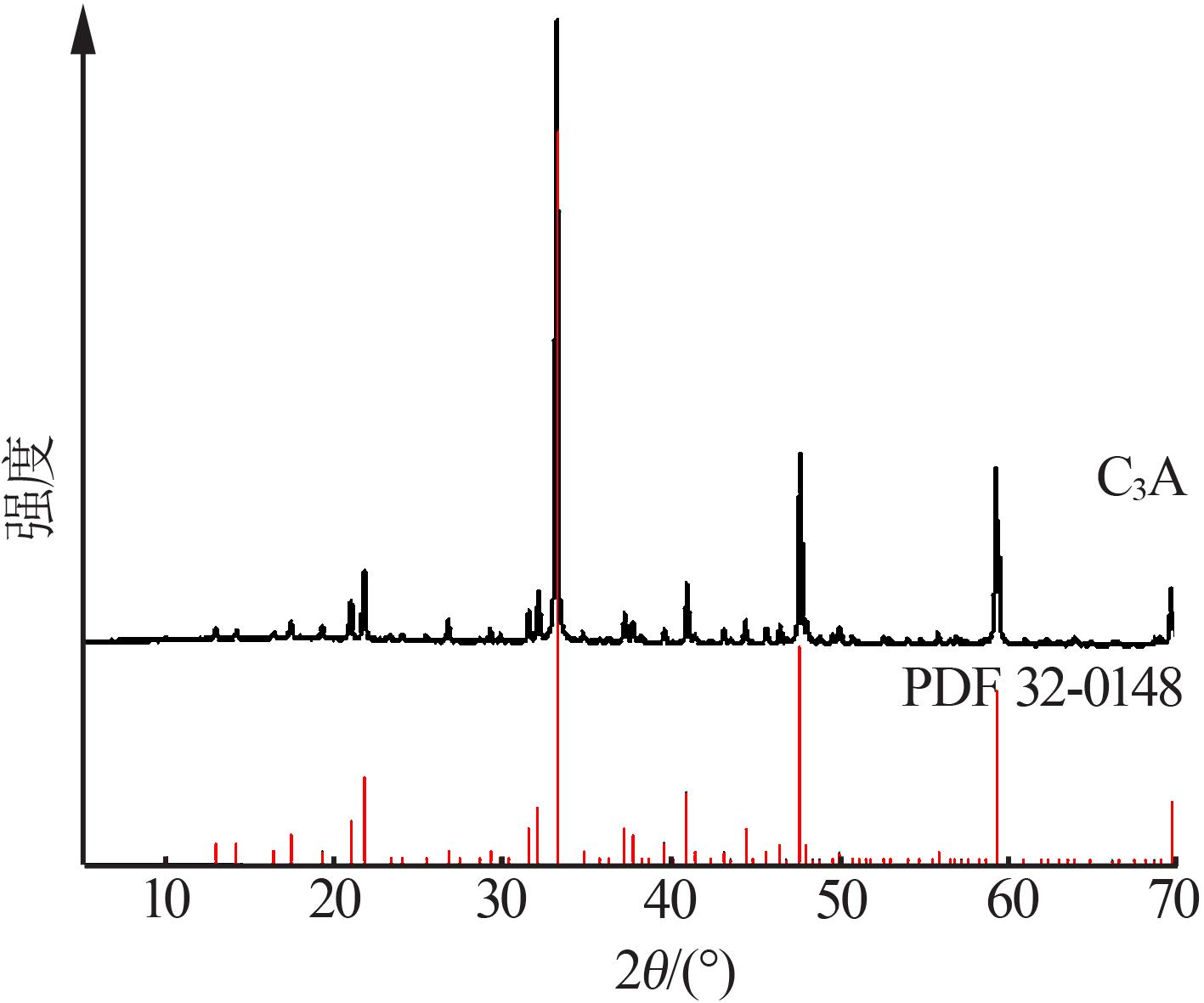
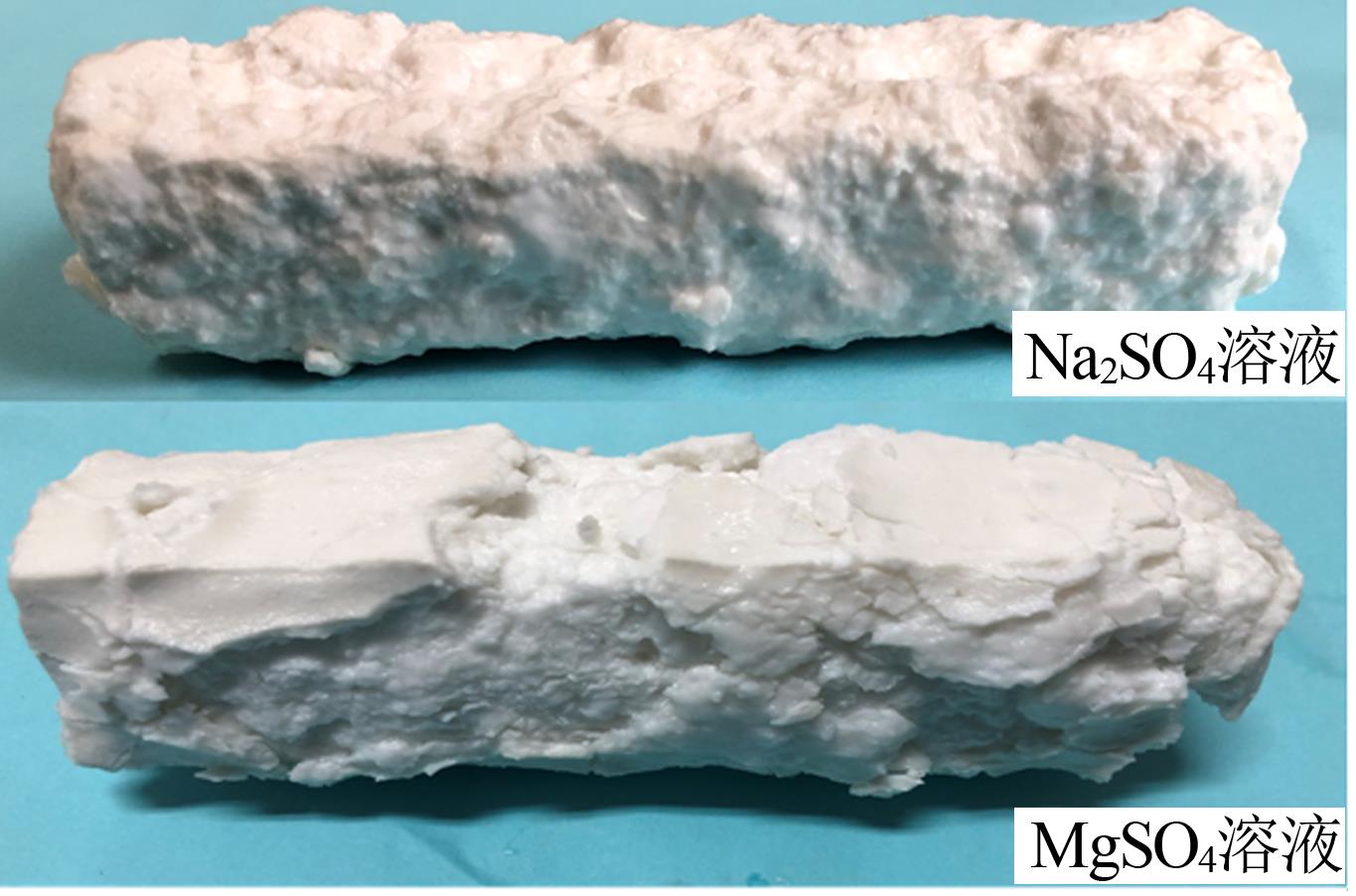
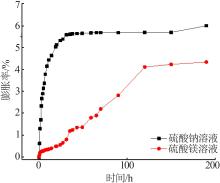
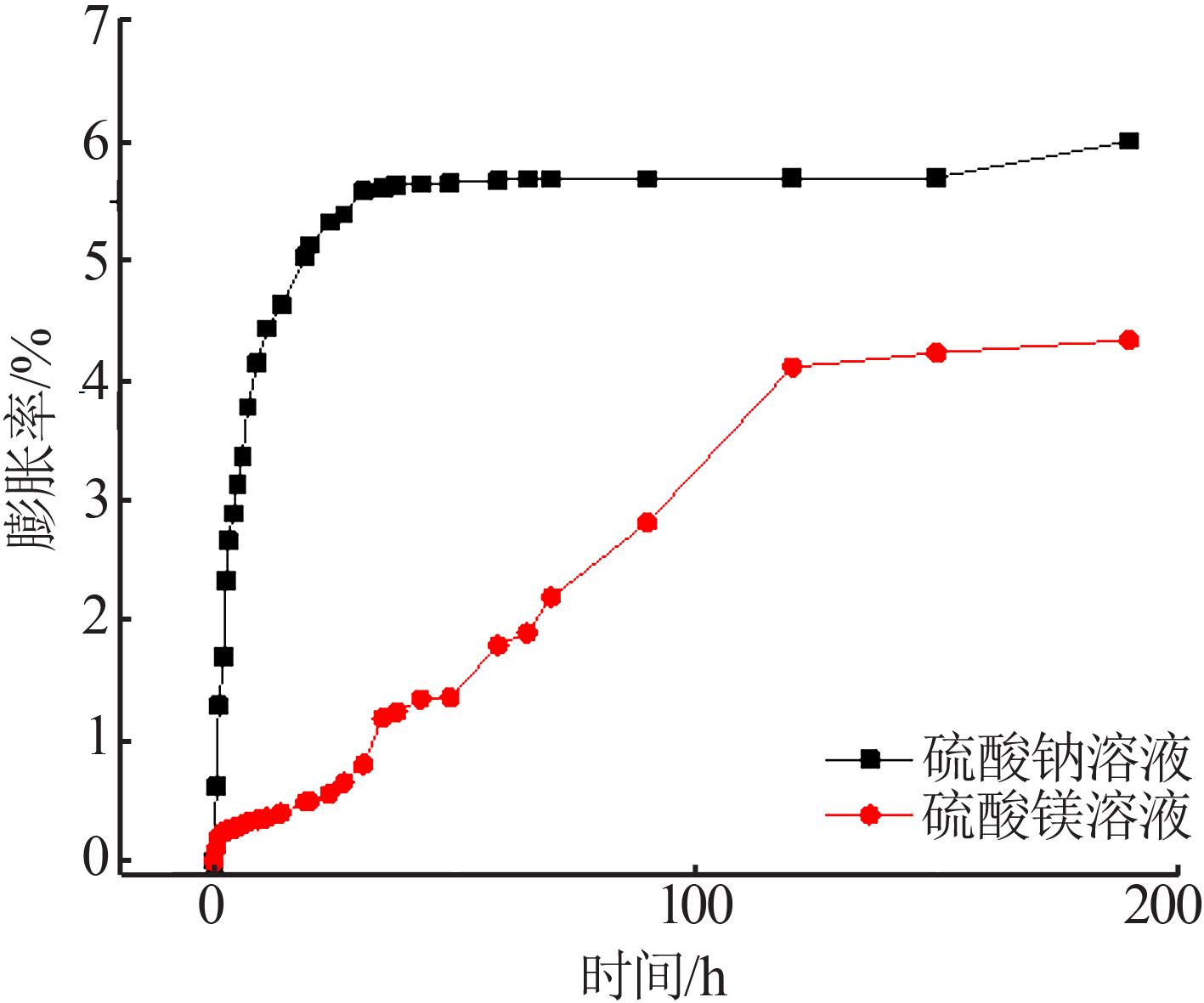
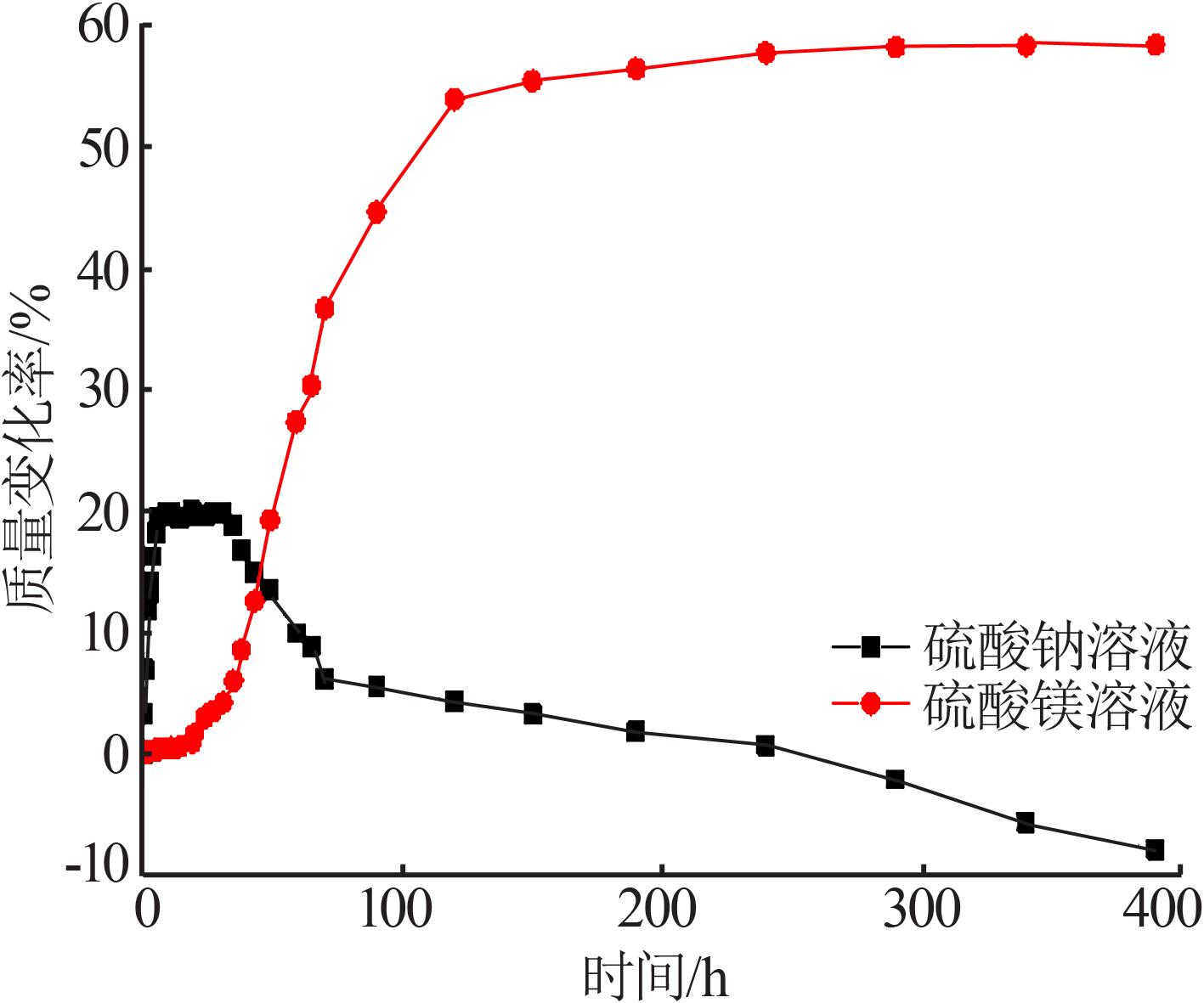
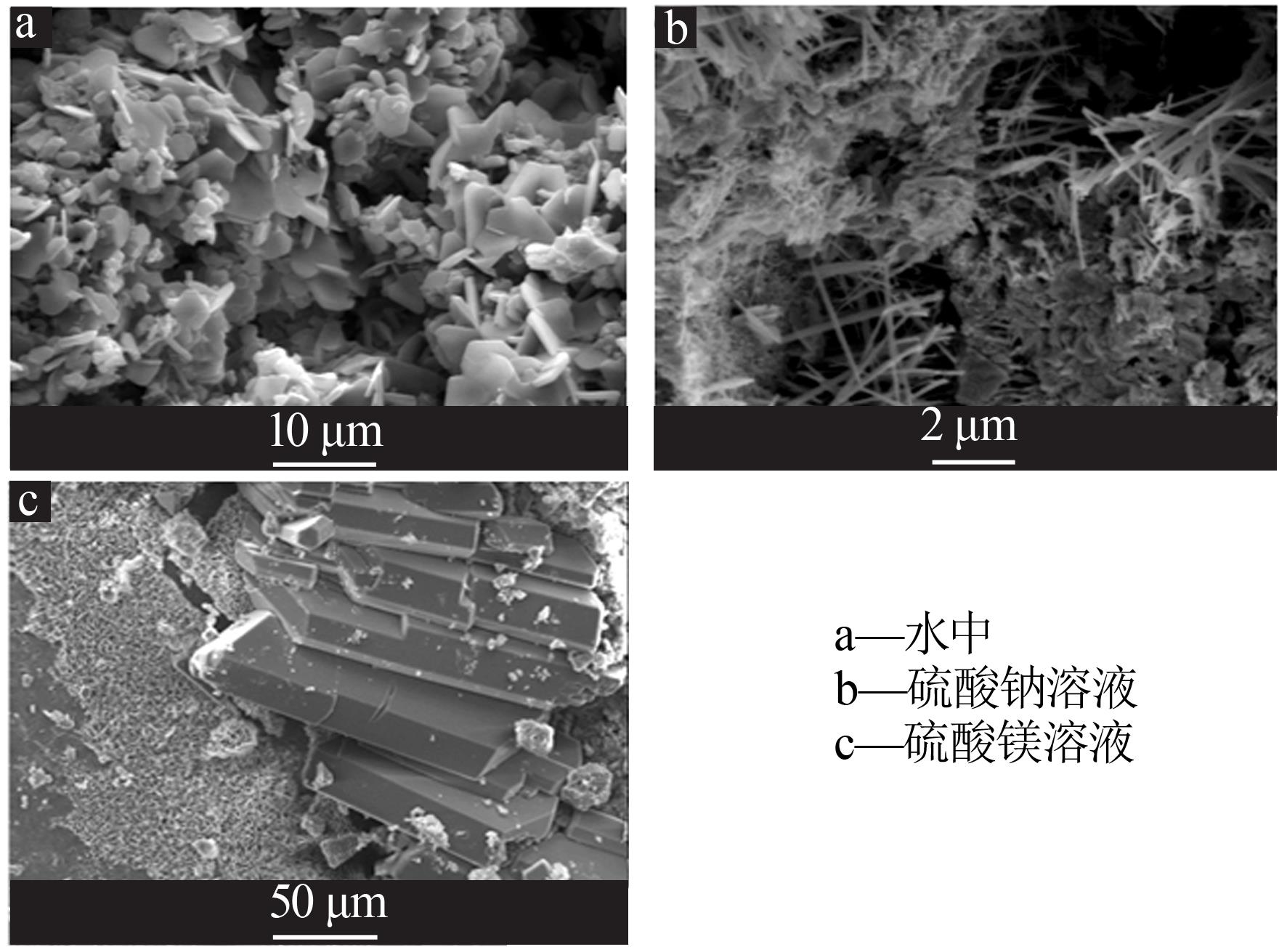
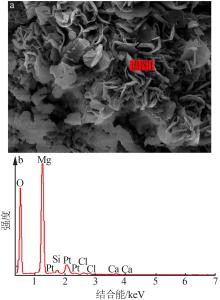
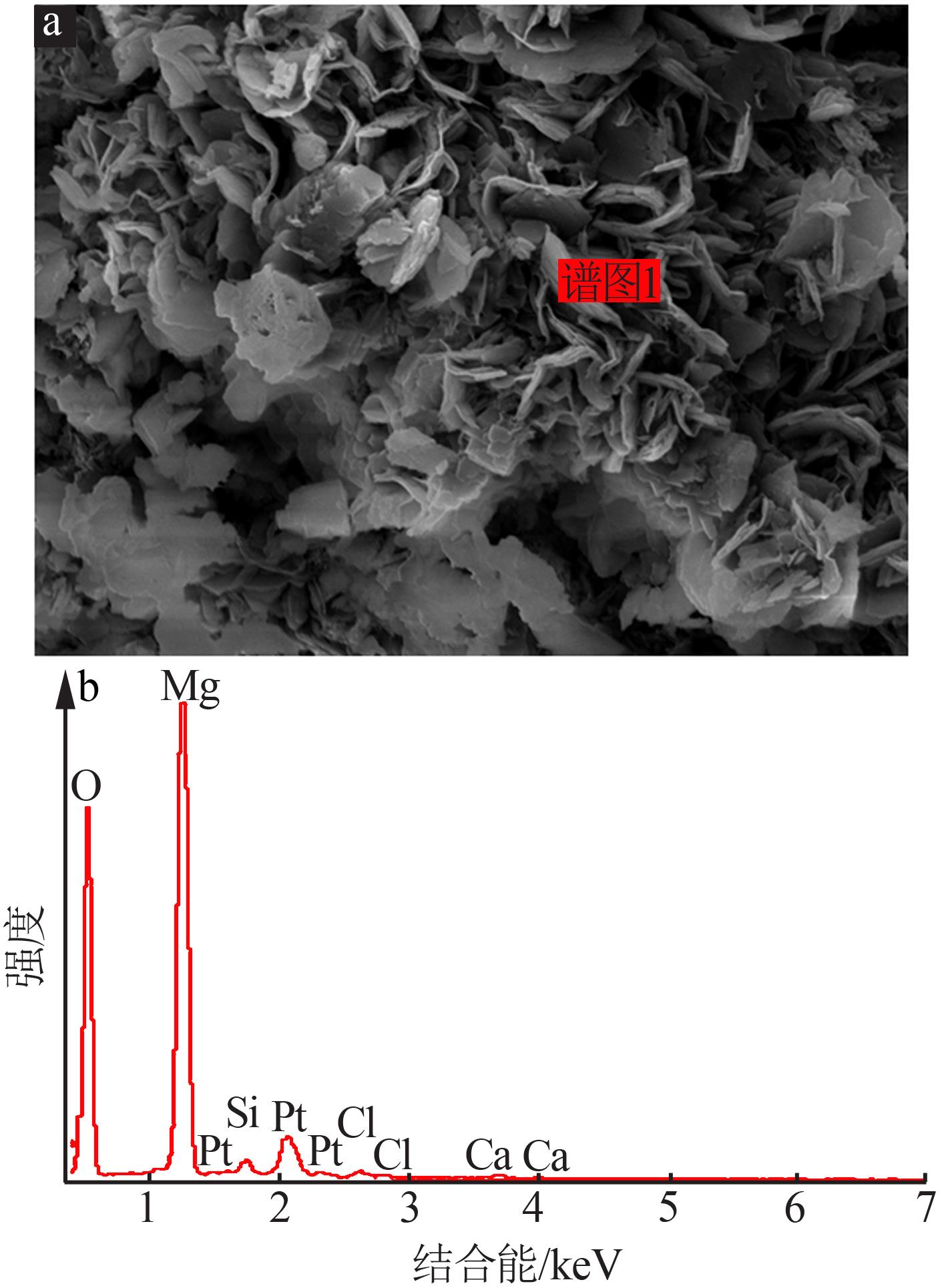
| [1] |
LIU Xiaowen, LI Jun, ZHOU Zhaoan, MAO Anzhang, ZHOU Aiqing.
Study on response surface methodology optimization of PAC for deep purification of fluorine ion in high concentration sodium sulfate solution
[J]. Inorganic Chemicals Industry, 2024, 56(6): 67-72.
|
| [2] |
LI Hongyuan, ZHANG Jianhua.
Study on removal process of total organic carbon from industrial waste salts by pyrolysis
[J]. Inorganic Chemicals Industry, 2024, 56(10): 95-102.
|
| [3] |
WEI Tianshun, JI Lijun, SHENG Yong, CHEN Kui, WU Yanyang, WU Bin.
Study on fractional crystallization process of ammonium sulfate and sodium sulfate in high salt wastewater
[J]. Inorganic Chemicals Industry, 2024, 56(1): 102-106.
|
| [4] |
ZHOU Zhaoan, LI Jun, LIU Xiaowen, ZHOU Aiqing, MAO Anzhang.
Study on carbonization and purification process of high COD industrial waste salt
[J]. Inorganic Chemicals Industry, 2023, 55(9): 100-105.
|
| [5] |
JIANG Demin, LI Shunmei, Li Qingqing, Chen Yuxin, LIU Daijun.
Hydrothermal synthesis of basic magnesium sulfate whiskers from magnesium hydroxide and magnesium sulfate heptahydrate
[J]. Inorganic Chemicals Industry, 2023, 55(12): 74-81.
|
| [6] |
LAI Xianrong, CHEN Zhouqin, SUN Hao, YANG Chao.
Pilot study on lithium extraction by adsorption from raw brine of magnesium sulfate subtype salt lakes in Tibet
[J]. Inorganic Chemicals Industry, 2023, 55(11): 86-92.
|
| [7] |
ZHENG Sanqiang,LI Xingbin,LUO Xingguo,WEI Chang,HUANG Xing,DENG Zhigan,LI Minting.
Production and control of water insoluble substance in sodium sulfate produced by NaCl-Na2SO4 co?production
[J]. Inorganic Chemicals Industry, 2022, 54(8): 90-95.
|
| [8] |
LIU Zhuang,AN Jimin,LI Yongjun,CHEN Xing,ZHAO Yigang,ZHAI Ruiguo.
Effect of impurity in alkaline washing oxidation process on crystal size of sodium sulfate
[J]. Inorganic Chemicals Industry, 2022, 54(4): 123-127.
|
| [9] |
Tian Haiying,Zhou Fu,Xu Chuan,Tu Mingjiang,Zhong Zhaozi,Liao Shiying,Li Shihong.
Application research of Li+,Na+,K+,SO42--H2O system phase diagram in MVR evaporation process
[J]. Inorganic Chemicals Industry, 2021, 53(8): 75-78.
|
| [10] |
YANG Zhuoying,YANG Fan,YI Meigui,XIANG Lan.
Rule of hydrothermal hydrolysis of Mg/Al-bearing TiOSO4 solution
[J]. Inorganic Chemicals Industry, 2021, 53(12): 113-116.
|
| [11] |
Wang Yanfei,Jiao Jian,Jiang Shuwan,Xu Shijie.
Study on nucleation kinetics of sodium sulfate-water system based on inverse solubility
[J]. Inorganic Chemicals Industry, 2021, 53(10): 41-46.
|
| [12] |
Zhang Bingbing,Ren Jianpo,Shen Zhihong,Jiang Zhiqiang.
Experimental study on tail gas absorption in synthesis of 2,3-pyridinedicarboxylic acid
[J]. Inorganic Chemicals Industry, 2020, 52(7): 74-76.
|
| [13] |
Liao Enxin,Chen Lifang,Zhang Zeya,Bai Fengxia.
Experimental study on preparing potassium sulfate by sodium sulfate and potassium chloride
[J]. Inorganic Chemicals Industry, 2020, 52(10): 106-109.
|
| [14] |
Hao Jiantang.
Study on producing anhydrous hydrogen fluoride by fluorosilicic acid and magnesium oxide and combined production of high quality magnesium sulfate
[J]. Inorganic Chemicals Industry, 2019, 51(8): 40-43.
|
| [15] |
Chen Xia, Bai Fengxia, Shi Yapeng, Dong Sicheng, Liao Enxin, Li Ying, Miao Shulan.
Experimental research of preparation of potassium sulfate from high-salt wastewater in coal chemical industry by double decomposition method
[J]. Inorganic Chemicals Industry, 2019, 51(6): 57-61.
|
 ),QI Daozheng1,CHEN Deng2
),QI Daozheng1,CHEN Deng2