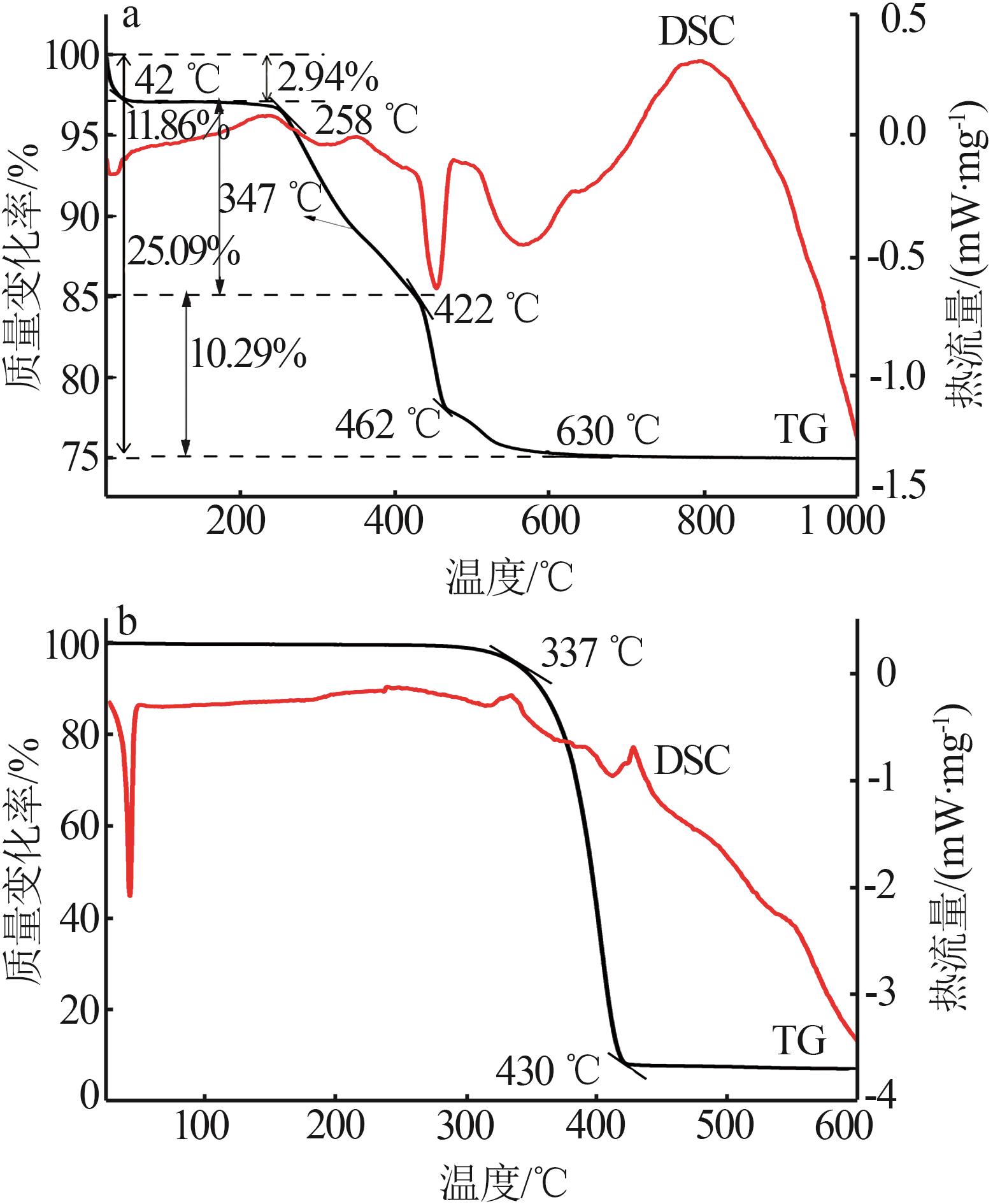
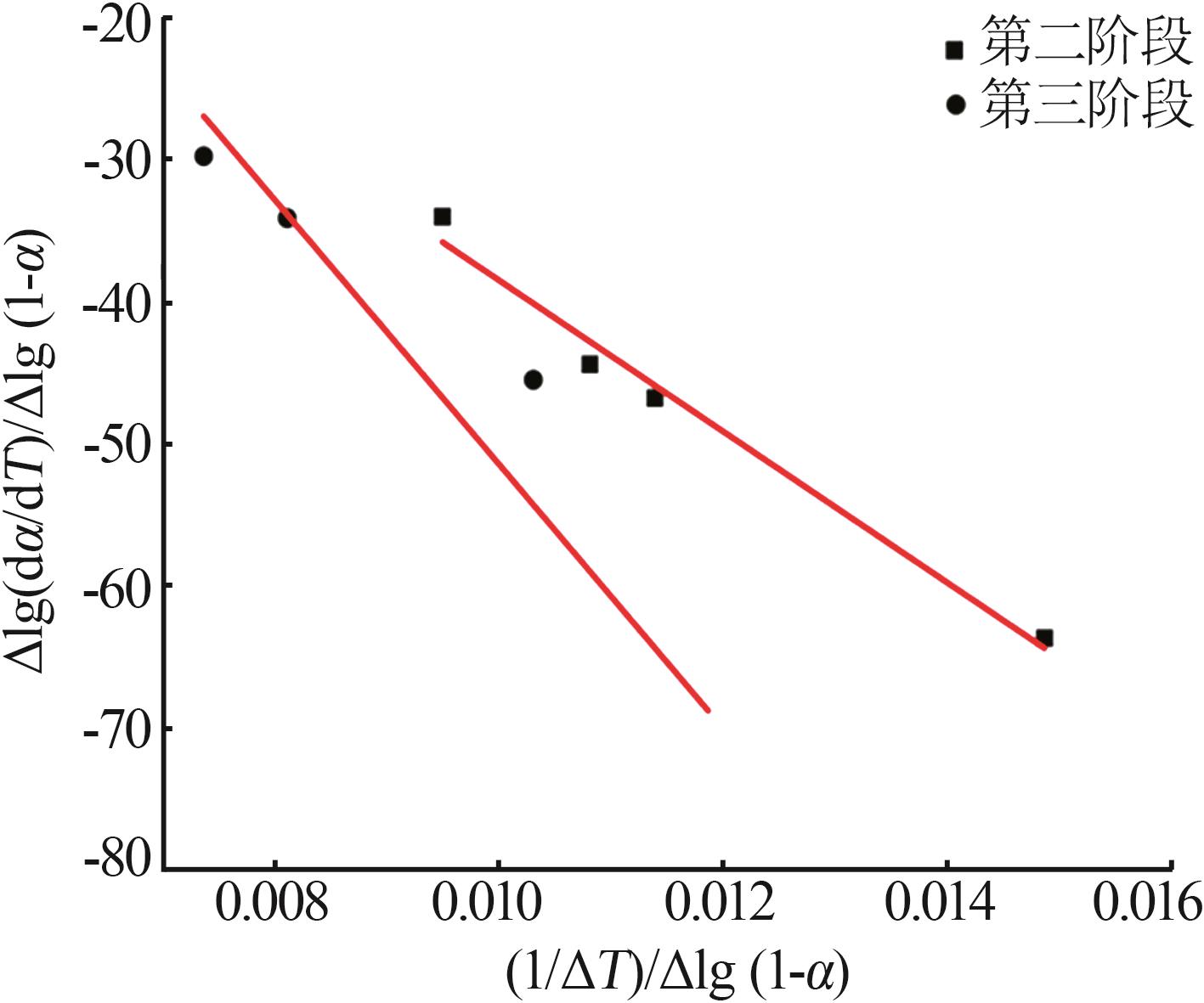
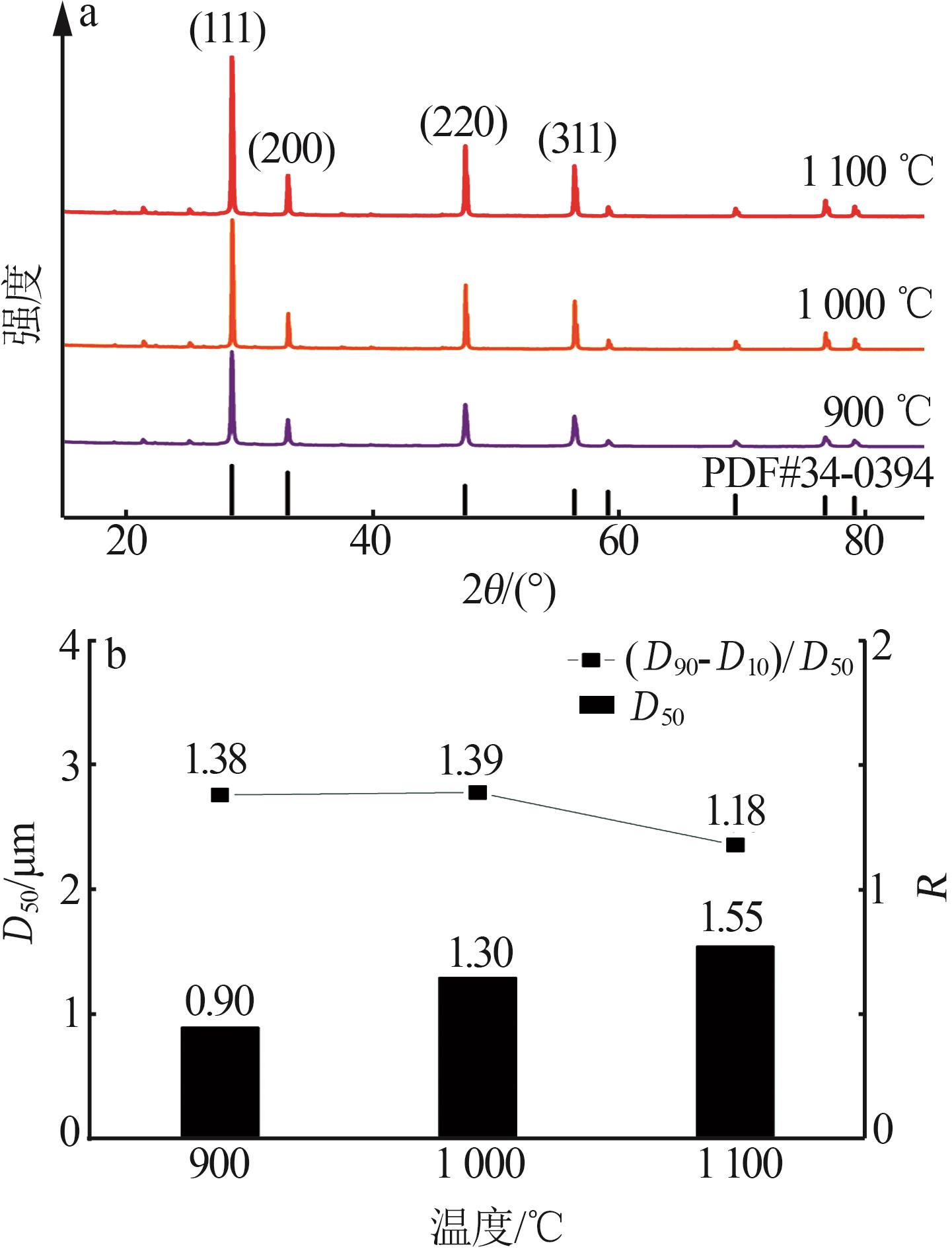
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (6): 77-84.doi: 10.19964/j.issn.1006-4990.2024-0313
• Research & Development • Previous Articles Next Articles
Study on thermal decomposition behaviour of ultrafine cerium oxide with reticulated framework
MENG Longhui1,2,3( ), HU Yanhong1,2,3(
), HU Yanhong1,2,3( ), YANG Guosheng4, ZHAO Yan4, ZHANG Xiaowei2,3, LIU Zhaogang2,3, WANG Ruifen3
), YANG Guosheng4, ZHAO Yan4, ZHANG Xiaowei2,3, LIU Zhaogang2,3, WANG Ruifen3
- 1. School of Materials Science and Engineering,Inner Mongolia University of Science and Technology,Baotou 014000,China
2. Key Laboratory of Rare Earth Hydrometallurgy and Light Rare Earth Applications,Inner Mongolia Autonomous Region,Baotou 014000,China
3. Key Laboratory of Green Extraction and Efficient Utilization of Light Rare Earth Resources,Ministry of Education,Baotou 014000,China
4. Baotou Tian-jiao Seimi RE Polishing Powder Co. ,Ltd. ,Baotou 014030,China
-
Received:2024-06-06Online:2025-06-10Published:2025-06-25 -
Contact:HU Yanhong E-mail:774505582@qq.com;bthyh@163.com
CLC Number:
Cite this article
MENG Longhui, HU Yanhong, YANG Guosheng, ZHAO Yan, ZHANG Xiaowei, LIU Zhaogang, WANG Ruifen. Study on thermal decomposition behaviour of ultrafine cerium oxide with reticulated framework[J]. Inorganic Chemicals Industry, 2025, 57(6): 77-84.
share this article
| 1 | 郝晓东,冯心怡,徐阳,等.二氧化铈纳米催化剂可控合成及超微观构效关系研究[J].无机盐工业,2022,54(3):7-17. |
| HAO Xiaodong, FENG Xinyi, XU Yang,et al.Study on controllable synthesis and ultramicroscopic structure-activity relationship of cerium oxides nanocatalysts[J].Inorganic Chemicals Industry,2022,54(3):7-17. | |
| 2 | WANG Xingzi, WANG Ning, ZHANG Zhenyu,et al.Controlled synthesis of triangular submicron-sized CeO2 and its polishing performance[J]. Materials,2024,17(9):2001. |
| 3 | 刘翠翠,李梅,胡艳宏,等.聚苯乙烯磺酸钠调控制备球状碳酸铈颗粒研究[J].无机盐工业,2012,44(8):13-15. |
| LIU Cuicui, LI Mei, HU Yanhong,et al.Research in preparation of spherical Ce2(CO3)3 particles under control of PSS[J].Inorganic Chemicals Industry,2012,44(8):13-15. | |
| 4 | JAVID H, AMIRI H, HASHEMI S F,et al.Sol-gel synthesis and cytotoxicity evaluation of selenium-doped cerium oxide nanoparticles for biomedical applications[J].Naunyn-Schmiedeberg′s Archives of Pharmacology,2024,397(5):3437-3447. |
| 5 | ALSHARIF N B, SAMU G F, SÁRINGER S,et al.Antioxidant colloids via heteroaggregation of cerium oxide nanoparticles and latex beads[J].Colloids and Surfaces B:Biointerfaces,2022,216:112531. |
| 6 | 周薇.光学透镜用铈基稀土抛光粉的制备研究[D].包头:内蒙古科技大学,2020. |
| ZHOU Wei.Study on preparation of cerium-based rare earth polishing powder for optical lens[D].Baotou:Inner Mongolia University of Science & Technology,2020. | |
| 7 | 贾慧灵,徐阳,吴锦绣,等.制备条件对二氧化铈稀土抛光粉性能的影响[J].稀土,2022,43(4):37-45. |
| JIA Huiling, XU Yang, WU Jinxiu,et al.Effect of preparation conditions on the properties of cerium oxide polishing powder[J].Chinese Rare Earths,2022,43(4):37-45. | |
| 8 | JANOŠ P, EDERER J, PILAŘOVÁ V,et al.Chemical mechanical glass polishing with cerium oxide:Effect of selected physico-chemical characteristics on polishing efficiency[J].Wear,2016,362:114-120. |
| 9 | MA Jiahui, XU Ning, LUO Yuxin,et al.Defect generation and morphology transformation mechanism of CeO2 particles prepared by molten salt method[J].Ceramics International,2023,49(3):4929-4943. |
| 10 | 贾红伟,贾艳虹,李斌,等.甲酸铈煅烧制备二氧化铈[J].核化学与放射化学,2022,44(3):347-355. |
| JIA Hongwei, JIA Yanhong, LI Bin,et al.Preparation of cerium dioxide by calcination of cerium formate[J].Journal of Nuclear and Radiochemistry,2022,44(3):347-355. | |
| 11 | 吴婷,李静,朱敏萱,等.湿固相机械法制备氧化铈抛光粉[J].稀土,2020,41(5):1-10. |
| WU Ting, LI Jing, ZHU Minxuan,et al.Preparation of ceria polishing powder by wet solid phase mechanical method[J].Chinese Rare Earths,2020,41(5):1-10. | |
| 12 | LI Mei, LIU Zhaogang, HU Yanhong,et al.Effects of the synthesis methods on the physicochemical properties of cerium dioxide powder[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2007,301(1/2/3):153-157. |
| 13 | CHAFIQ M, CHAOUIKI A, KO Y G.Recent advances in multifunctional reticular framework nanoparticles:A paradigm shift in materials science road to a structured future[J].Nano-Micro Letters,2023,15(1):213. |
| 14 | 李学舜.稀土碳酸盐制备铈基稀土抛光粉的研究[D].沈阳:东北大学,2008. |
| LI Xueshun.Study on preparation of cerium-based rare earth polishing powder from rare earth carbonate[D].Shenyang:Northeastern University,2008. | |
| 15 | 周强,武斌,陈葵,等.磷尾矿热分解动力学机理与煅烧工艺研究[J].无机盐工业,2023,55(3):47-54. |
| ZHOU Qiang, WU Bin, CHEN Kui,et al.Study on thermal decomposition kinetic mechanism and calcination process of phosphorus tailings[J].Inorganic Chemicals Industry,2023,55(3):47-54. | |
| 16 | 卢博,刘宜强,蒋威,等.大颗粒氧化镧粉体制备研究进展[J].稀土,2022,43(6):119-124. |
| LU Bo, LIU Yiqiang, JIANG Wei,et al.Research progress on preparation of large-grained lanthanum oxide powder[J].Chinese Rare Earths,2022,43(6):119-124. | |
| 17 | 梅燕,陈文娟,陈学安.氟化镧铈抛光粉的焙烧制备及抛光性能研究[J].功能材料,2024,55(1):1010-1015. |
| MEI Yan, CHEN Wenjuan, CHEN Xue′an.Preparation and polishing performance of lanthanum cerium fluoride polishing powder[J].Journal of Functional Materials,2024,55(1):1010-1015. | |
| 18 | 范娜,陈传东,于健飞,等.基于氟硅混酸制备稀土抛光粉及其抛光性能研究[J].中国稀土学报,2024,42(2):261-267. |
| FAN Na, CHEN Chuandong, YU Jianfei,et al.Preparation of rare earth polishing powder based on fluorosilicate mixed acid and its polishing performance[J].Journal of the Chinese Society of Rare Earths,2024,42(2):261-267. | |
| 19 | 李学舜.稀土抛光粉的生产及应用[J].中国稀土学报,2002,20(5):392-397. |
| LI Xueshun.Preparation and application of rare earth polishing powder[J].Journal of the Chinese Rare Earth Society,2002,20(5):392-397. | |
| 20 | 杨国胜,边雪,崔凌霄,等.氯化铈溶液喷雾焙烧制备纳米氧化铈实心粉体的研究[J].稀土,2017,38(1):72-78. |
| YANG Guosheng, BIAN Xue, CUI Lingxiao,et al.Study on preparation of nano cerium oxide powder by spray roasting of cerium chloride solution[J].Chinese Rare Earths,2017,38(1):72-78. | |
| 21 | ZHAN Guang, YU Junxia, XU Zhigao,et al.Kinetics of thermal decomposition of lanthanum oxalate hydrate[J].Transactions of Nonferrous Metals Society of China,2012,22(4):925-934. |
| 22 | 丁勇,冯晓锐,鲁相杰,等.羰基镍粉焙烧制备高纯度微米氧化亚镍粉体的研究[J].无机盐工业,2022,54(4):119-122,140. |
| DING Yong, FENG Xiaorui, LU Xiangjie,et al.Study on preparation of high purity micron nickel oxide powder by roasting carbonyl nickel powder[J].Inorganic Chemicals Industry,2022,54(4):119-122,140. | |
| 23 | 饶明璐,高国华,陈金发,等.超细碳酸钇热分解行为及焙烧工艺的研究[J].中国稀土学报,2022,40(2):276-285. |
| RAO Minglu, GAO Guohua, CHEN Jinfa,et al.Thermal decomposition behavior and roasting process of ultrafine yttrium carbonate[J].Journal of the Chinese Society of Rare Earths,2022,40(2):276-285. | |
| 24 | 朱钰方,方莹,张少明.低温固相反应制备氧化锌微粉研究[J].南京工业大学学报(自然科学版),2002,24(6):70-73. |
| ZHU Yufang, FANG Ying, ZHANG Shaoming.Preparation of ultrafine ZnO by solid state reaction under low temperature[J].Journal of Nanjing University(Natural Science Edition),2002,24(6):70-73. | |
| 25 | 张超,谢永纯,喻航,等.焙烧时间对喷雾干燥制备LiMnPO4/C多孔微球的影响[J].无机盐工业,2018,50(2):24-28. |
| ZHANG Chao, XIE Yongchun, YU Hang,et al.Influence of calcination time on preparation porous LiMnPO4/C microspheres by spray drying process[J].Inorganic Chemicals Industry,2018,50(2):24-28. |
| [1] | SHI Yunpeng, GUO Ze, ZHANG Hanquan, LU Manman. Study on treatment of ammonia nitrogen wastewater by roasted phosphorus tailings [J]. Inorganic Chemicals Industry, 2025, 57(3): 94-100. |
| [2] | MAO Shize. Study on microwave roasting-activated coal gangue to improve sulfate corrosion resistance of concrete [J]. Inorganic Chemicals Industry, 2025, 57(3): 86-93. |
| [3] | WANG Li, YAN Xiaohong, KE Guopeng, MEI Jiaxin, CHEN Mingyue, LIU Jingyong. Research progress of ceramsite prepared by sintering heavy metal-contaminated soil [J]. Inorganic Chemicals Industry, 2025, 57(1): 27-35. |
| [4] | ZENG Yijun, JIANG Ziwen, JIAN Chengzong, QUAN Xuejun. Study on deep extraction of chromium from calcium-free roasting slag of chromite ore [J]. Inorganic Chemicals Industry, 2025, 57(1): 90-96. |
| [5] | ZHANG Yaqi, CHEN Xiping, SUN Ningning. Study on preparation process of cryolite from anode carbon slag by catalytic decarburization [J]. Inorganic Chemicals Industry, 2024, 56(3): 91-97. |
| [6] | LUO Wenbo, LI Heng, LÜ Jun, YANG Linguang, ZHAO Xingfan, LONG Xiao. Study on recovery of silicon and aluminum from industrial silicon slag [J]. Inorganic Chemicals Industry, 2023, 55(9): 94-99. |
| [7] | WANG Benlei, ZHANG Guisheng, JIANG Lingyun, LI Chen, WANG Pengfei, LI Jixia, ZANG Jiazhong. Study on preparation process of rhodium trichloride by roasting recovery technology from waste rhodium solution of carbonyl synthesis [J]. Inorganic Chemicals Industry, 2023, 55(6): 104-108. |
| [8] | CHEN Yujue, ZHANG Liangjun, KUANG Huan, JIANG Manwen, XIAO Li. Study on roasting and recovery process of waste lithium iron phosphate powder with sodium bisulfate [J]. Inorganic Chemicals Industry, 2023, 55(3): 113-117. |
| [9] | GUO Ze, ZHANG Pengfei, YANG Fan, ZHANG Hanquan, LU Manman. Preparation of β-hemihydrate gypsum by phosphogypsum roasting [J]. Inorganic Chemicals Industry, 2023, 55(10): 106-113. |
| [10] | DING Yong,FENG Xiaorui,LU Xiangjie,GAO Xiaoqin,FENG Yan,HE Yan. Study on preparation of high purity micron nickel oxide powder by roasting carbonyl nickel powder [J]. Inorganic Chemicals Industry, 2022, 54(4): 119-122. |
| [11] | ZENG Yanjie,XU Tongfei,HUANG Ziliang,CHEN Zhipeng,LIU Xudong,YI Meigui. Study on removal of calcium impurities from magnesium waste residue by roasting-hydration complexing [J]. Inorganic Chemicals Industry, 2022, 54(11): 118-123. |
| [12] | Sun Shuangshuang,Zhong Jianchu,Wang Hongzhi. Study on desiliconization process of copper smelting slag [J]. Inorganic Chemicals Industry, 2021, 53(9): 83-87. |
| [13] | Yan Zhenzong,Wang Dong. Study on rotary kiln reduction roasting production process of barite in Tianzhu of Guizhou [J]. Inorganic Chemicals Industry, 2021, 53(4): 73-75. |
| [14] | Shang Fangyu,Su Xuetong,Shang Suying. Study on recovery of barium from barium slag by chlorination roasting-water leaching process [J]. Inorganic Chemicals Industry, 2021, 53(3): 65-67. |
| [15] | Qing Penghui,Dong Yuming,Wang Xingrun,Zhu Kaisheng,Niu Renjie,Meng Jingjuan,Chen Xiaohong,Chen Huixia,Zhang Hongling,Xu Hongbin. Chromium extraction and detoxification of processing residue from lime-free roasting process of chromite ore [J]. Inorganic Chemicals Industry, 2020, 52(6): 63-67. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||