Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (12): 56-62.doi: 10.19964/j.issn.1006-4990.2024-0618
• Research & Development • Previous Articles Next Articles
Study on removal process of chlorine from fluorosilicic acid by ozonidation
CHEN Yi1( ), LI Tianxiang1, LIU Songlin2, SUI Yanfeng2(
), LI Tianxiang1, LIU Songlin2, SUI Yanfeng2( ), LI Baiyu2, ZHU Jing1(
), LI Baiyu2, ZHU Jing1( )
)
- 1. School of Chemistry and Chemical Engineering,Guizhou University,Guiyang 550025,China
2. State Key;Laboratory of Green and Effciient Development of Phosphorus Resources,Guiyang 550014,China
-
Received:2024-11-20Online:2025-12-10Published:2025-08-01 -
Contact:SUI Yanfeng, ZHU Jing E-mail:2359062773@qq.com;1223260229@qq.com;jzhu1@gzu.edu.cn
CLC Number:
Cite this article
CHEN Yi, LI Tianxiang, LIU Songlin, SUI Yanfeng, LI Baiyu, ZHU Jing. Study on removal process of chlorine from fluorosilicic acid by ozonidation[J]. Inorganic Chemicals Industry, 2025, 57(12): 56-62.
share this article
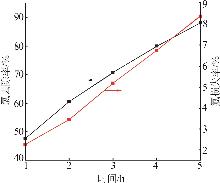
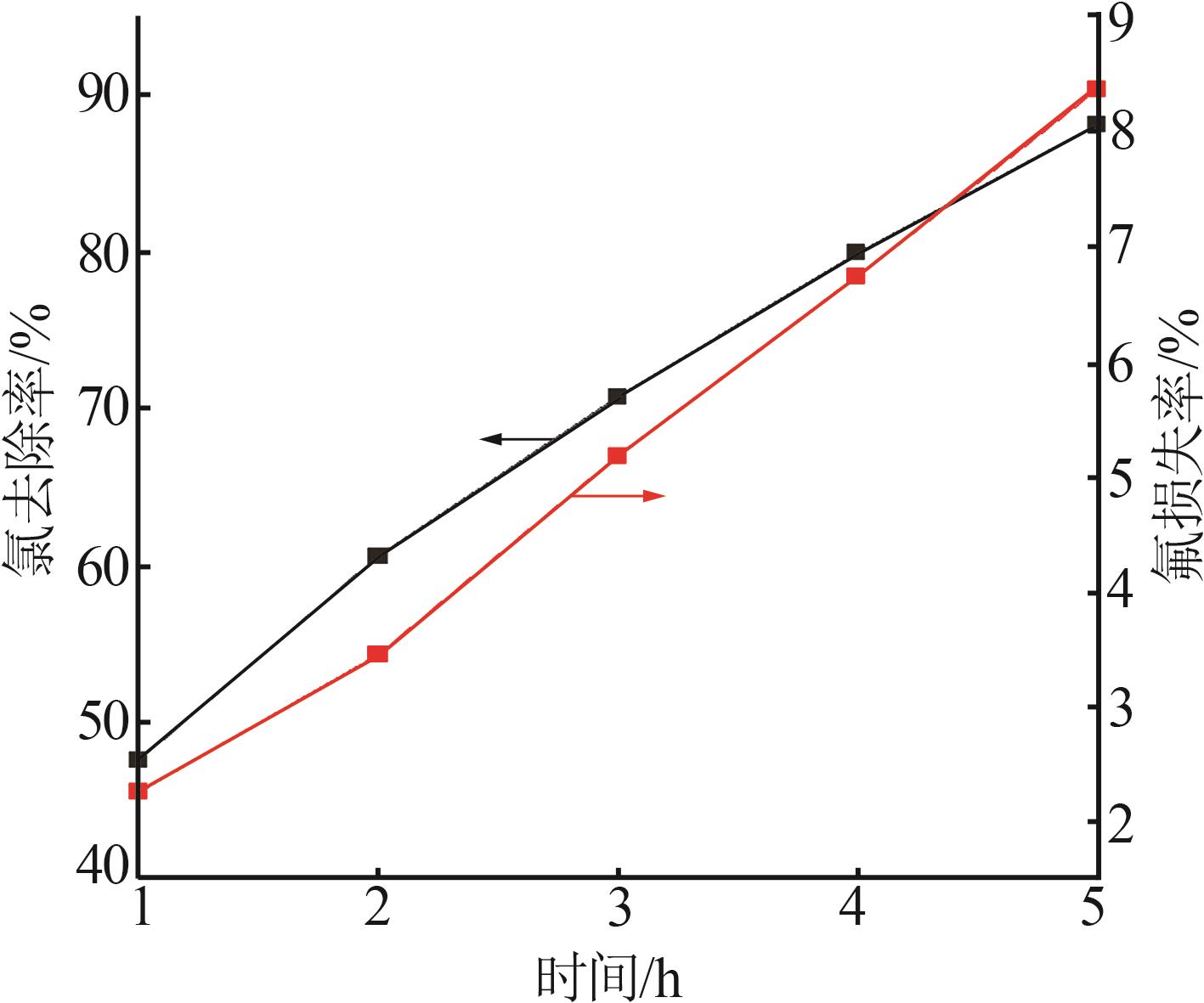
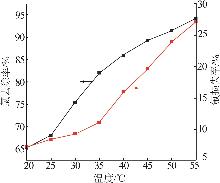
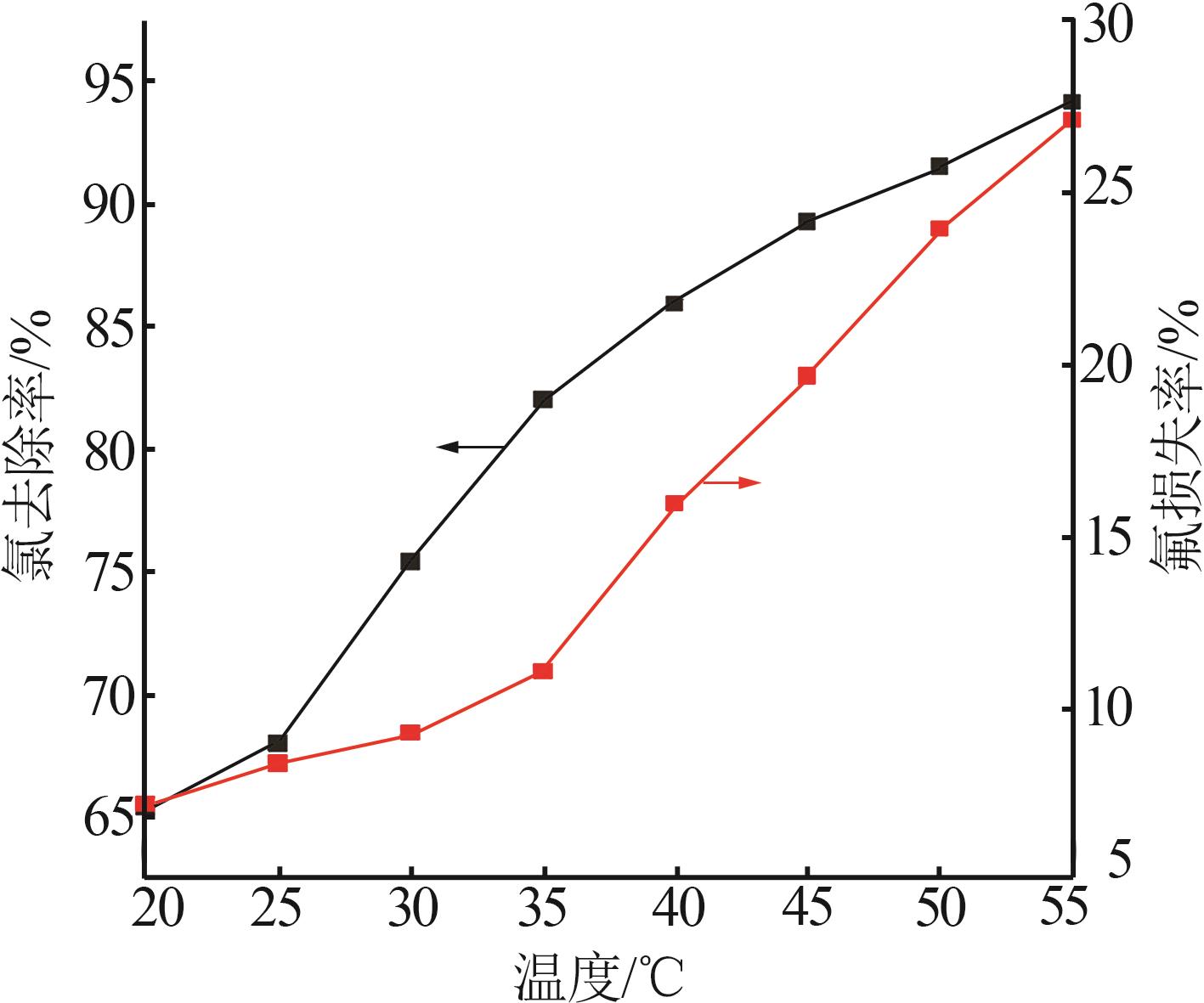
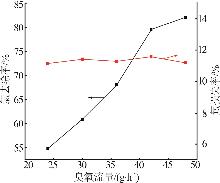
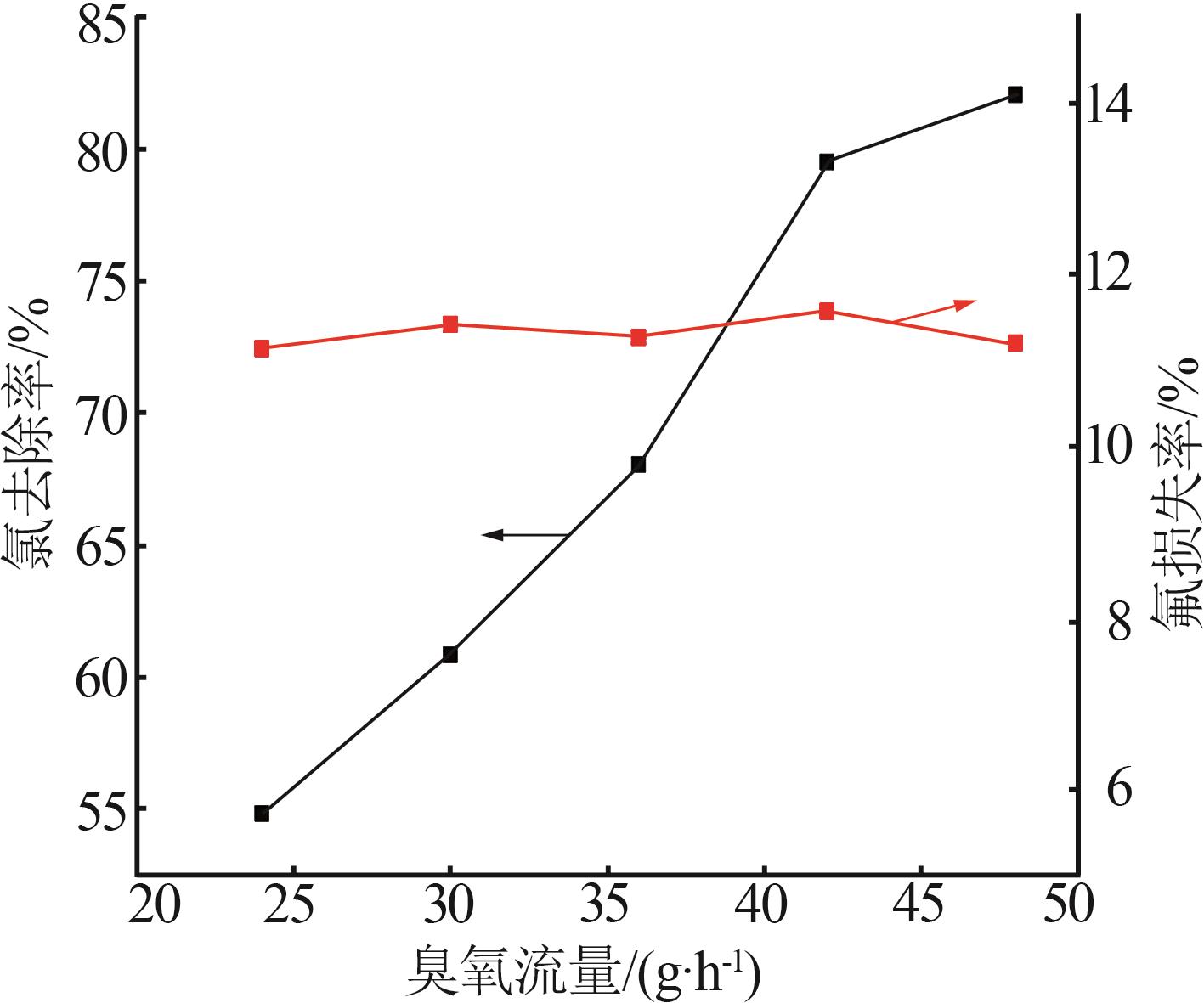
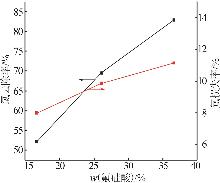
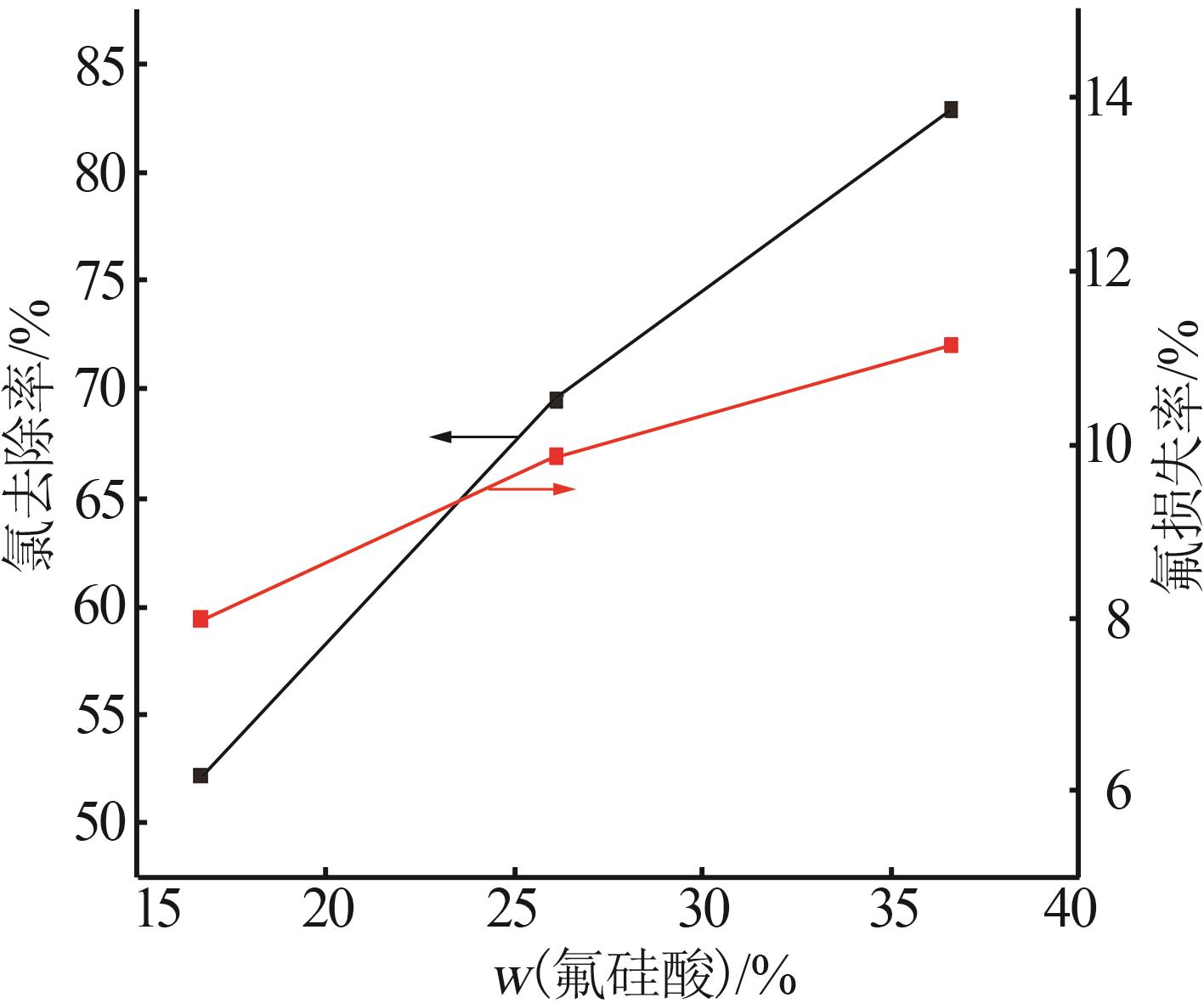
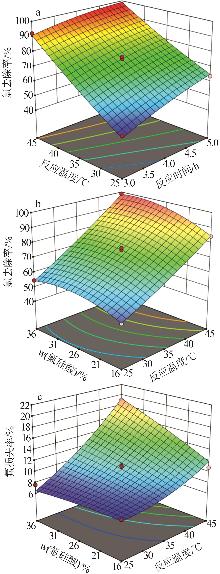
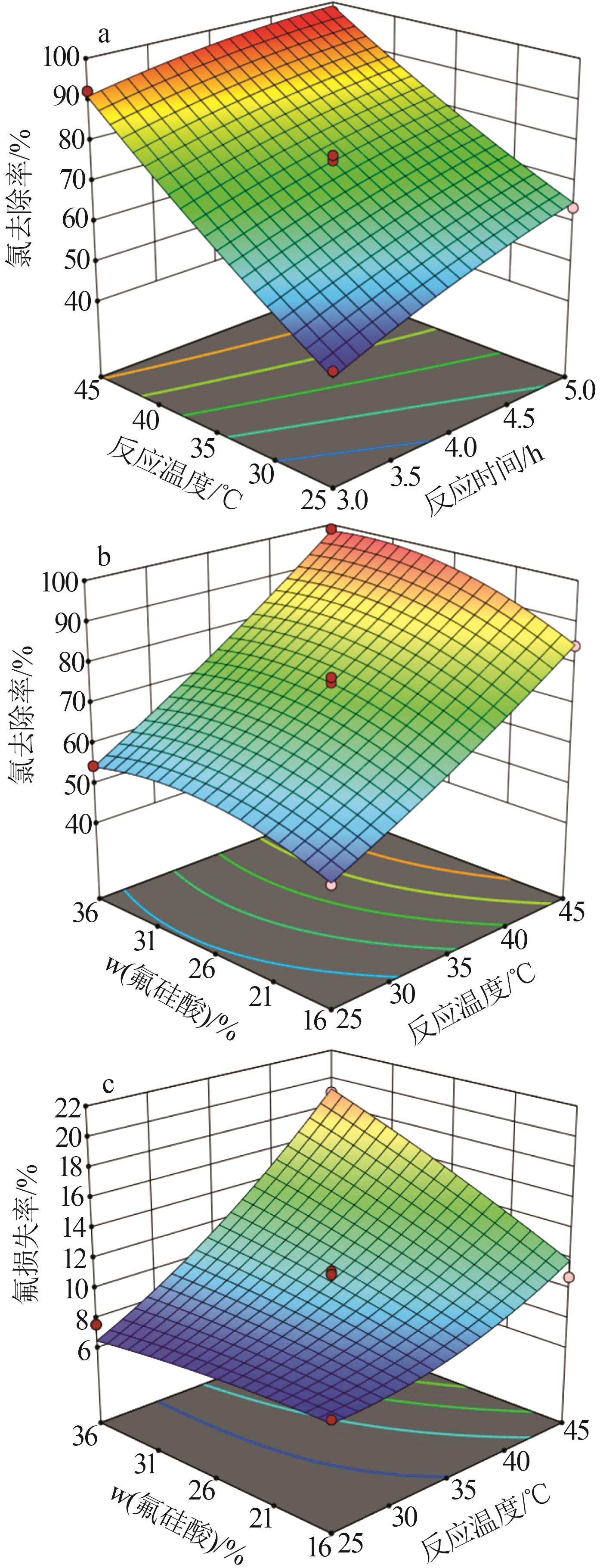
Table 2
Experimental design and results of BBD"
| 编号 | A/h | B/℃ | C/% | 氯去除率/% | 氟损失率/% |
|---|---|---|---|---|---|
| 1 | 4 | 45 | 36.00 | 98.79 | 10.80 |
| 2 | 4 | 35 | 26.00 | 76.73 | 9.63 |
| 3 | 5 | 25 | 26.00 | 63.71 | 12.01 |
| 4 | 4 | 35 | 26.00 | 76.73 | 9.63 |
| 5 | 3 | 25 | 26.00 | 47.50 | 7.30 |
| 6 | 5 | 45 | 26.00 | 97.55 | 20.35 |
| 7 | 3 | 35 | 16.00 | 58.43 | 10.93 |
| 8 | 5 | 35 | 36.00 | 83.09 | 14.45 |
| 9 | 4 | 35 | 26.00 | 73.47 | 11.01 |
| 10 | 3 | 45 | 26.00 | 92.20 | 18.76 |
| 11 | 4 | 45 | 16.00 | 84.33 | 19.02 |
| 12 | 4 | 35 | 26.00 | 74.05 | 10.98 |
| 13 | 4 | 25 | 36.00 | 54.56 | 7.84 |
| 14 | 4 | 25 | 16.00 | 49.32 | 7.56 |
| 15 | 5 | 35 | 16.00 | 70.77 | 17.78 |
| 16 | 3 | 35 | 36.00 | 62.93 | 9.83 |
| 17 | 4 | 35 | 26.00 | 75.34 | 11.21 |
Table 3
Results of regression statistical analysis of chloride removal rate"
| 方差来源 | 平方和 | 自由度 | 均方 | F | P | 显著性 |
|---|---|---|---|---|---|---|
| 相关系数(R2) 0.993 5 | ||||||
| 模型 | 3 832.07 | 9 | 425.79 | 118.96 | <0.000 1 | 极显著 |
| A | 365.31 | 1 | 365.31 | 102.06 | <0.000 1 | 极显著 |
| B | 3 111.82 | 1 | 3 111.82 | 869.40 | <0.000 1 | 极显著 |
| C | 166.71 | 1 | 166.71 | 46.58 | 0.000 2 | 极显著 |
| AB | 29.48 | 1 | 29.48 | 8.24 | 0.024 0 | 显著 |
| AC | 15.29 | 1 | 15.29 | 4.27 | 0.077 6 | |
| BC | 21.25 | 1 | 21.25 | 5.94 | 0.045 0 | 显著 |
| A² | 9.28 | 1 | 9.28 | 2.59 | 0.151 4 | |
| B² | 8.98 | 1 | 8.98 | 2.51 | 0.157 2 | |
| C² | 104.19 | 1 | 104.19 | 29.11 | 0.001 0 | 极显著 |
| 残差 | 25.05 | 7 | 3.58 | |||
| 失拟项 | 16.06 | 3 | 5.35 | 2.38 | 0.210 5 | 不显著 |
| 纯误差 | 9.00 | 4 | 2.25 | |||
| 总离差 | 3 857.13 | 16 | ||||
Table 4
Results of regression statistical analysis of fluoride loss rate"
| 方差来源 | 平方和 | 自由度 | 均方 | F | P | 显著性 |
|---|---|---|---|---|---|---|
| 相关系数(R2) 0.961 1 | ||||||
| 模型 | 271.86 | 9 | 30.21 | 19.21 | 0.000 4 | 极显著 |
| A | 39.47 | 1 | 39.47 | 25.10 | 0.001 5 | 极显著 |
| B | 146.38 | 1 | 146.38 | 93.09 | <0.000 1 | 极显著 |
| C | 19.13 | 1 | 19.13 | 12.16 | 0.010 2 | 显著 |
| AB | 2.43 | 1 | 2.43 | 1.55 | 0.253 5 | |
| AC | 1.24 | 1 | 1.24 | 0.79 | 0.403 4 | |
| BC | 18.06 | 1 | 18.06 | 11.49 | 0.011 6 | 显著 |
| A² | 38.60 | 1 | 38.60 | 24.55 | 0.001 6 | 极显著 |
| B² | 4.96 | 1 | 4.96 | 3.15 | 0.119 0 | |
| C² | 0.31 | 1 | 0.31 | 0.20 | 0.669 4 | |
| 残差 | 11.01 | 7 | 1.57 | |||
| 失拟项 | 8.50 | 3 | 2.83 | 4.52 | 0.089 6 | 不显著 |
| 纯误差 | 2.51 | 4 | 0.63 | |||
| 总离差 | 282.87 | 16 | ||||
| [1] | 刘海霞.氟硅酸综合利用工艺技术研究进展[J].无机盐工业,2017,49(3):9-13. |
| LIU Haixia.Research progress in technology of comprehensive utilization of fluosilicic acid[J].Inorganic Chemicals Industry,2017,49(3):9-13. | |
| [2] | 王睿哲,朱静,李天祥.磷肥副产氟硅酸综合利用研究现状与展望[J].无机盐工业,2018,50(12):9-12. |
| WANG Ruizhe, ZHU Jing, LI Tianxiang.Research status and progress of comprehensive utilization of fluosilicic acid from phosphate fertilizer plant[J].Inorganic Chemicals Industry,2018,50(12):9-12. | |
| [3] | 彭向龙,刘松林,隋岩峰,等.环己醇萃取浓缩氟硅酸的工艺研究[J].应用化工,2023,52(3):739-742. |
| PENG Xianglong, LIU Songlin, SUI Yanfeng,et al.Study on the technology of concentrating fluorosilicic acid by extraction[J].Applied Chemical Industry,2023,52(3):739-742. | |
| [4] | 彭向龙,刘松林,隋岩峰,等.溶剂萃取技术在磷矿加工工艺中的应用[J].化学工业与工程,2024,41(4):147-159. |
| PENG Xianglong, LIU Songlin, SUI Yanfeng,et al.Application of solvent extraction technology in phosphate ore processing technology[J].Chemical Industry and Engineering,2024,41(4):147- 159. | |
| [5] | 哀鹏鹏,彭朝凯,刘成龙,等.湿法磷酸脱氟工艺的研究现状与发展方向[J].湿法冶金,2024,43(1):9-14. |
| AI Pengpeng, PENG Zhaokai, LIU Chenglong,et al.Research status and development direction of defluorination technology of wet-process phosphoric acid[J].Hydrometallurgy of China,2024,43(1):9-14. | |
| [6] | WANG Zirui, AN Xiaowei, WANG Peifen,et al.Removal of high concentration of chloride ions by electrocoagulation using aluminium electrode[J].Environmental Science and Pollution Research,2023,30(17):50567-50581. |
| [7] | HU Yihang, WANG Haibei, WANG Yufang,et al.Simultaneous removal of fluorine and chlorine from zinc sulfate solution in iron precipitation process[J].Journal of Sustainable Metallurgy,2018,4(1):95-102. |
| [8] | ZHAO Minghu, WANG Shixing, ZHANG Libo.Removal of chlorine from zinc sulfate solution:A review[J].Environmental Science and Pollution Research,2022,29(42):62839-62850. |
| [9] | ZHENG Qian, XU Hengyue, YAO Yancai,et al.Cobalt single-atom reverse hydrogen spillover for efficient electrochemical water dissociation and dechlorination[J].Angewandte Chemie International Edition,2024,63(19):e202401386. |
| [10] | 孙凤娟,刘万超,闫琨,等.离子交换树脂法去除脱硫废水中氯离子的研究[J].无机盐工业,2019,51(6):45-48. |
| SUN Fengjuan, LIU Wanchao, YAN Kun,et al.Removal of chloride ion from desulfurization wastewater by ion-exchange resin[J].Inorganic Chemicals Industry,2019,51(6):45-48. | |
| [11] | SABBAGHI S, MALEKI R, SHARIATY-NIASSAR M,et al.Modeling chloride ion removal from gas condensates by nanofiltration membrane separation[J].International Journal of Chemical & Environmental Engineering,2012,3(2):80-85. |
| [12] | XIAO Qian, LI Wanbin, XIE Shujie,et al.Ultrafast complete dechlorination enabled by ferrous oxide/graphene oxide catalytic membranes via nanoconfinement advanced reduction[J].Nature Communications,2024,15:9607. |
| [13] | 金若菲,滕丽曼,周集体.高氯离子废水CODCr测定的方法研究[J].环境污染与防治,2003,25(5):310-311. |
| JIN Ruofei, TENG Liman, ZHOU Jiti.Determination of CODCr in high Cl- wastewater[J].Environmental Pollution & Control,2003,25(5):310-311. | |
| [14] | ABATE C, SCHEETZ B E.Aqueous phase equilibria in the system CaO-Al2O3-CaCl2-H2O:The significance and stability of Friedel′s salt[J].Journal of the American Ceramic Society,1995,78(4):939-944. |
| [15] | WU Xuelian, LIU Zhongqing, LIU Xu.Chloride ion removal from zinc sulfate aqueous solution by electrochemical method[J].Hydrometallurgy,2013,134/135:62-65. |
| [16] | CARMONA M, PÉREZ A, DE LUCAS A,et al.Removal of chloride ions from an industrial polyethylenimine flocculant shifting it into an adhesive promoter using the anion exchange resin Amberlite IRA-420[J].Reactive and Functional Polymers,2008,68(8):1218-1224. |
| [17] | DU Minggui, CHEN Qing, GAO Weiting,et al.Selective removal of chloride from the adipate formation bath in foil industry by diffusion dialysis[J].Separation and Purification Technology,2020,230:115871. |
| [18] | 邢铁瀚,余思泽,赵冬汁,等.微反应器在臭氧氧化水处理技术中的应用研究进展[J].化工环保,2024,44(6):751-760. |
| XING Tiehan, YU Size, ZHAO Dongzhi,et al.Research progress of microreactors in ozone-based advanced oxidation processes for water treatment[J].Environmental Protection of Chemical Industry,2024,44(6):751-760. | |
| [19] | 吴雅琴,熊威,邹方远,等.臭氧技术处理含盐对氯苯酚废水的研究[J].水处理技术,2022,48(1):108-111,117. |
| WU Yaqin, XIONG Wei, ZOU Fangyuan,et al.Research on ozonation to treat sodium salt p-chlorophenol wastewater[J].Technology of Water Treatment,2022,48(1):108-111,117. |
| [1] | WANG Danqin, LIAN Yicheng, DU Xin, YAN Feifei, LIU Wanchao, REN Wurong. Research progress on treatment technology for fluorine-containing solid wastes of electrolytic aluminum [J]. Inorganic Chemicals Industry, 2025, 57(6): 9-17. |
| [2] | DENG Zhi, LONG Bingwen, ZHANG Yi, DENG Fuli, DAI Yafen, LIU Shaofeng, DING Yigang. Study on wet synthesis process of aluminum fluoride from fluosilicic acid by-product of phosphorus chemical industry [J]. Inorganic Chemicals Industry, 2025, 57(3): 71-77. |
| [3] | LI Shuai, LI Tianxiang, ZHU Jing, LIU Songlin. Study on purification process of sodium fluoride [J]. Inorganic Chemicals Industry, 2024, 56(9): 90-97. |
| [4] | ZHU Zuoqiao, SHI Mengyuan, MAO Rui, GUO Haining, SU Yuao. Study on treatment process of metallurgical fluorine-containing wastewater by coagulant sedimentation method [J]. Inorganic Chemicals Industry, 2024, 56(4): 118-124. |
| [5] | WANG Jianping, XUE Xujin, XUE Fengfeng. Study on new process of preparing high-purity aluminium fluoride from fluorosilicic acid [J]. Inorganic Chemicals Industry, 2024, 56(3): 86-90. |
| [6] | YANG Yong, CHEN Haiyang, LI Yukun, FAN Zongliang, LI Guixian, DUAN Zhiqiang, ZHAO Yu, WANG Dongliang. Progress of process development and industrial application for anhydrous hydrogen fluoride and aluminum fluoride [J]. Inorganic Chemicals Industry, 2023, 55(9): 17-25. |
| [7] | WANG Song, ZHANG Jianbin, SHEN Yüfang, QIN Shuhong, WANG Shengqiang, ZHANG Jingbing. Study on preparation process of high-purity zinc oxide by dechlorination of zinc oxide dust [J]. Inorganic Chemicals Industry, 2023, 55(11): 47-52. |
| [8] | ZHANG Lizhen,ZHANG Yongxing,WU Zhaoyang,ZHANG Xiufeng,TAN Xiumin. Experimental study on removal of water-soluble phosphorus and water-soluble fluorine from phosphogypsum [J]. Inorganic Chemicals Industry, 2022, 54(4): 40-45. |
| [9] | CHANG Wei,LIU Honghui,JIANG Xuguang. Study on dechlorination and heavy metal leaching characteristics of MSWI fly ash during water-washing [J]. Inorganic Chemicals Industry, 2022, 54(3): 113-118. |
| [10] | Zhang Xiaoxia. Study on preparation process of silicon tetrafluoride from fluosilicic acid and electric lime [J]. Inorganic Chemicals Industry, 2021, 53(9): 88-91. |
| [11] | Xie Tangfeng,Chen Ruokui,Cai Luorong,Wang Ming,Jiang Kuailiang. Determination of fluorine and chlorine in leaching solution in waste lithium battery by ion chromatography [J]. Inorganic Chemicals Industry, 2021, 53(7): 101-104. |
| [12] | Ou Jian,Li Tianxiang,Sui Yanfeng,Wu Qiang,Huang Linchuan,Zhu Jing. Review of treatment and resource recovery of industrial fluoride-containing waste gas [J]. Inorganic Chemicals Industry, 2021, 53(5): 39-46. |
| [13] | Wang Yuexiang,Shao Lanyan,Xu Tiannan,Cai Caihong,Yuan Junsheng,Zhang Yingwu. Study on existing speciation and deep dechlorination of chlorine in waste incineration fly ash [J]. Inorganic Chemicals Industry, 2021, 53(5): 78-83. |
| [14] | Qin Ganghua,Chen Biao,Feng Xiangdong,Chen Hui,Sun Qing,Zhang Jian,Sheng Jiawei. Research on dechlorination from dry ash of coal-fired power plants [J]. Inorganic Chemicals Industry, 2021, 53(10): 104-107. |
| [15] | Cao Weiwen,Li Jun,Chen Ming,Yu Yinrong,Liao Xiaoting. Study on agglomeration process during crystallization of calcium hydrogen phosphate and its main affecting impurity thereof [J]. Inorganic Chemicals Industry, 2021, 53(1): 50-53. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||