Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (10): 41-48.doi: 10.19964/j.issn.1006-4990.2024-0564
• Research & Development • Previous Articles Next Articles
Study on indoor evaporation and elemental behaviors of deep brine from Mahai region in Qaidam Basin
SHI Lijie1,2,3( ), ZHANG Xiufeng1,2,3(
), ZHANG Xiufeng1,2,3( ), ZHANG Lizhen1,2,3, YI Yuejun1,2,3, TAN Xiumin1,2,3
), ZHANG Lizhen1,2,3, YI Yuejun1,2,3, TAN Xiumin1,2,3
- 1. Zhengzhou Institute of Multipurpose Utilization of Mineral Resources,CAGS,Zhengzhou 450006,China
2. China National Engineering Research Center for Utilization of Industrial Minerals,Zhengzhou 450006,China
3. Key Laboratory of Polymetallic Ores′ Evaluation and Utilization,NMR,Zhengzhou 450006,China
-
Received:2024-10-25Online:2025-10-10Published:2024-12-05 -
Contact:ZHANG Xiufeng E-mail:shilijie@mail.cgs.gov.cn;zh200318@126.com
CLC Number:
Cite this article
SHI Lijie, ZHANG Xiufeng, ZHANG Lizhen, YI Yuejun, TAN Xiumin. Study on indoor evaporation and elemental behaviors of deep brine from Mahai region in Qaidam Basin[J]. Inorganic Chemicals Industry, 2025, 57(10): 41-48.
share this article
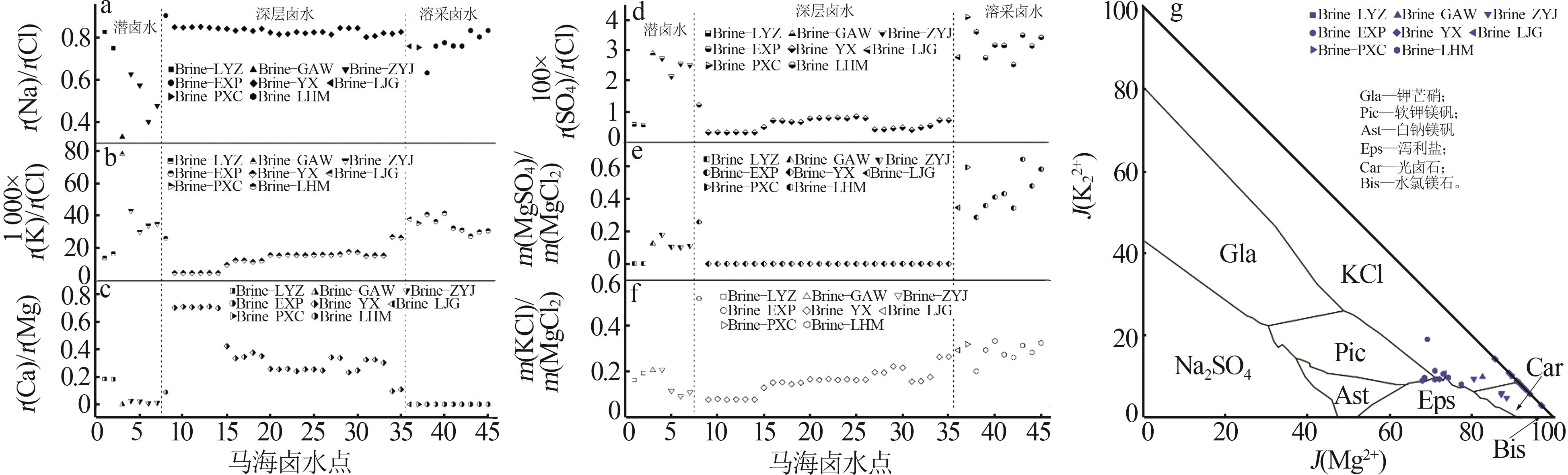
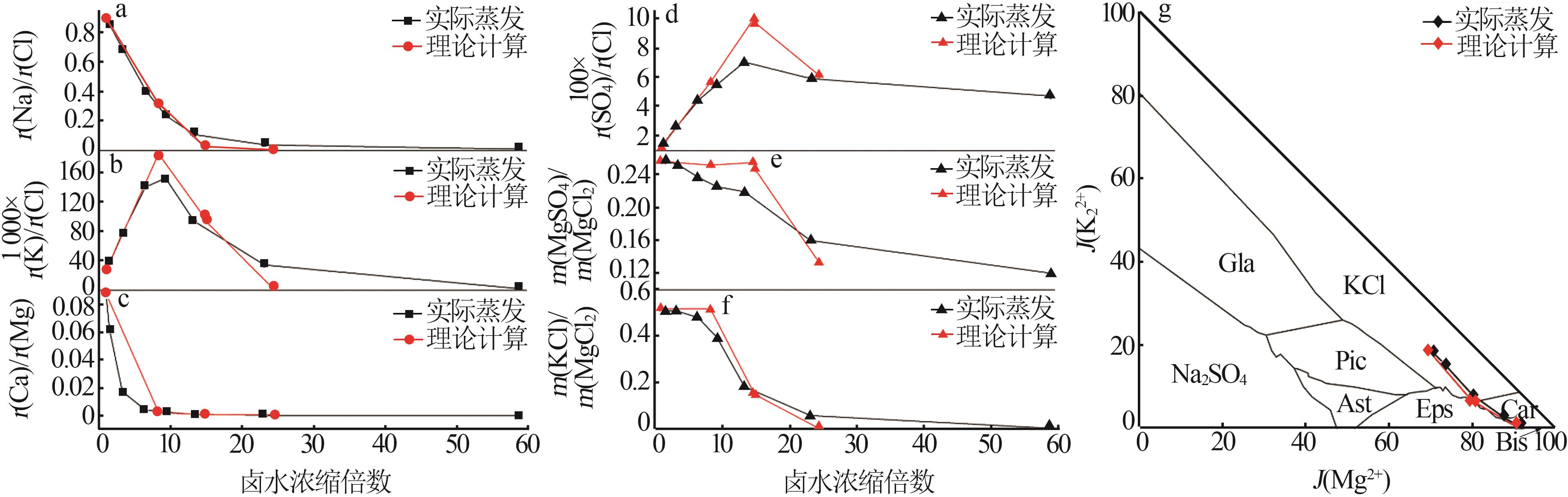
Table 2
Theoretical evaporation results of Mahai deep brine at 25 ℃"
| 阶段 | 编号 | 计算工艺参数 | 液、固相质量分数/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 成卤率/% | 析盐率/% | 蒸失率/% | Na+ | K+ | Mg2+ | Ca2+ | Cl- | SO42- | |||
| 石盐段 | LE-1 | 12.01 | 21.76 | 66.23 | 3.66 | 3.64 | 4.11 | 0.017 | 17.78 | 2.74 | |
| SE-1 | 38.78 | 0.00 | 0.00 | 0.330 | 59.81 | 0.78 | |||||
| 钾石盐段 | LE-2 | 6.81 | 23.40 | 69.79 | 0.32 | 1.96 | 7.24 | 0.009 | 17.86 | 4.84 | |
| SE-2 | 25.44 | 18.53 | 0.00 | — | 56.03 | — | |||||
| 钾混盐段 | LE-3 | 6.69 | 23.45 | 69.86 | 0.31 | 1.86 | 7.33 | 0.009 | 18.03 | 4.75 | |
| SE-3 | 2.83 | 17.88 | 5.79 | — | 20.58 | 22.88 | |||||
| 光卤石段 | LE-4 | 4.10 | 24.83 | 71.07 | 0.06 | 0.03 | 9.00 | 0.012 | 20.58 | 3.42 | |
| SE-4 | 1.31 | 8.94 | 8.82 | — | 26.35 | 12.91 | |||||
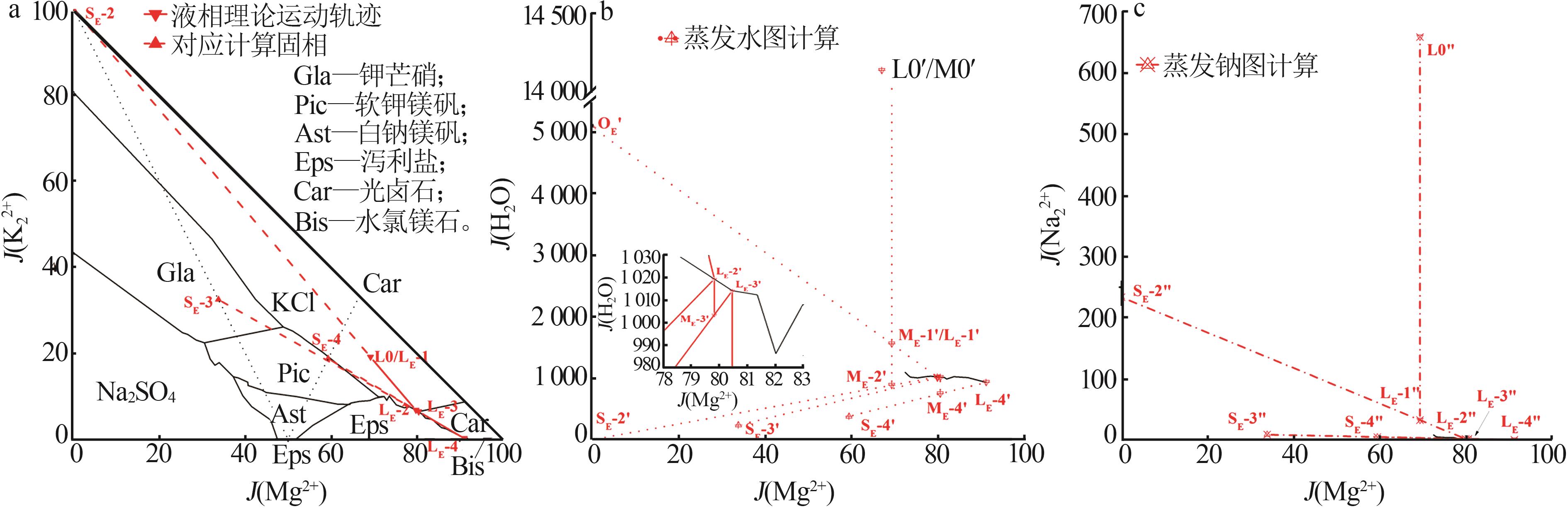
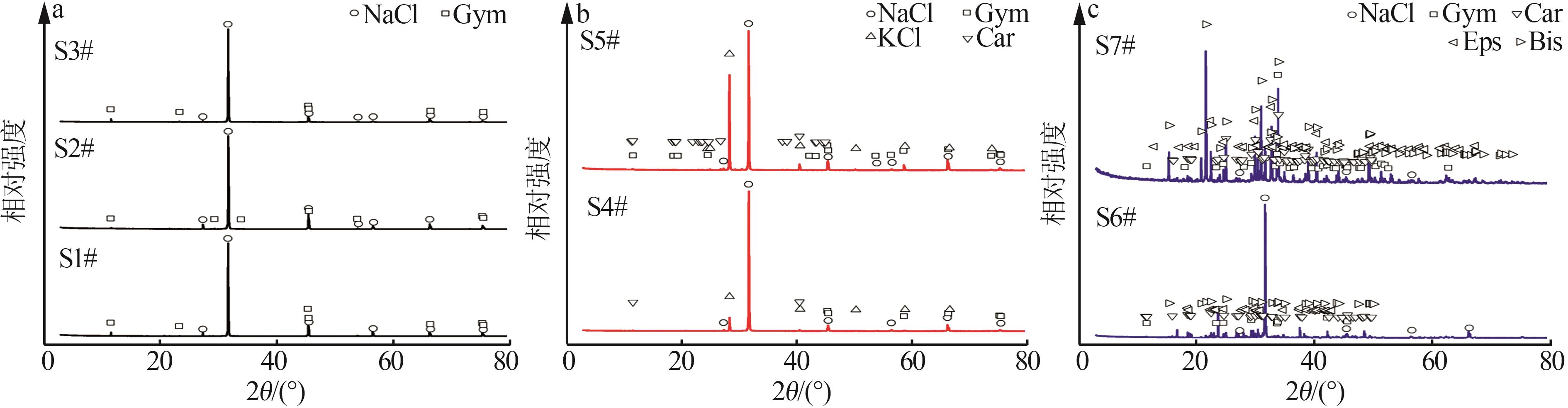
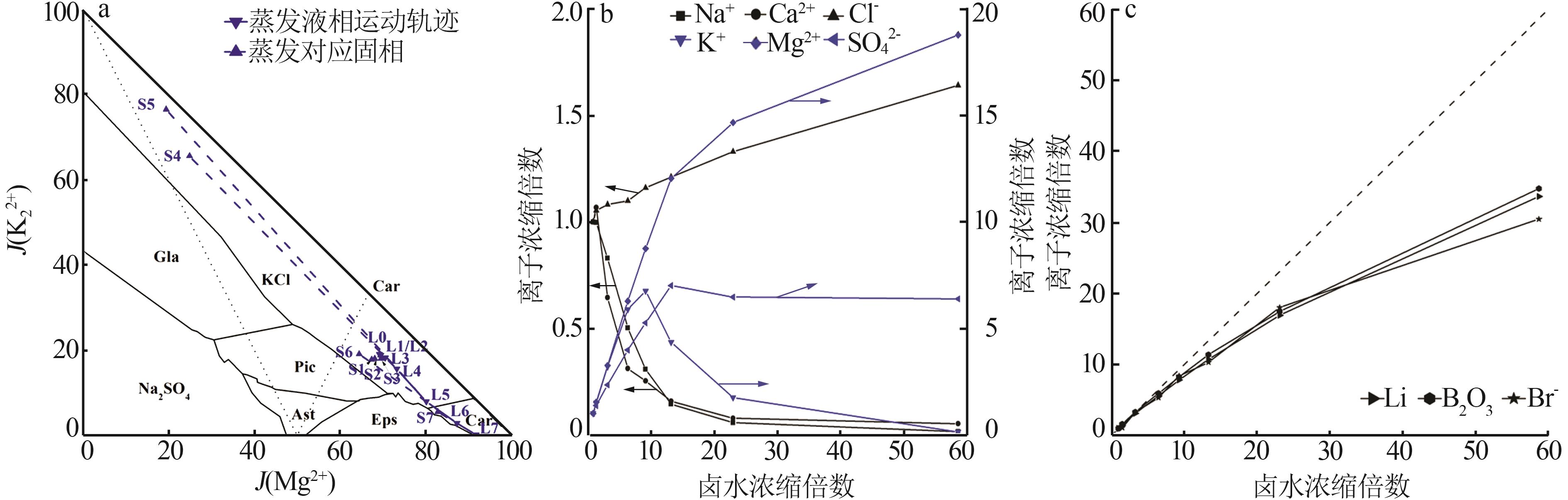
Table 3
Experimental indoor evaporation results of Mahai deep brine"
| 编号 | 工艺参数 | 液相质量浓度/(g·L-1),固相质量分数/% | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
成卤 率/% | 析盐 率/% | 蒸失 率/% | ρ/ (g·mL-1) | pH | Na+ | K+ | Mg2+ | Ca2+ | Cl- | SO42- | B2O3 | Li+ | Br- | ||
| L0 | 100.00 | 0.00 | 0.00 | 1.208 | 6.97 | 107.26 | 5.28 | 5.96 | 0.86 | 183.02 | 6.04 | 0.11 | 5.72×10-3 | 9.12×10-3 | |
| L1 | 66.94 | 9.04 | 24.02 | 1.210 | 6.83 | 107.16 | 7.92 | 9.10 | 0.92 | 193.16 | 8.28 | 0.18 | 8.20 | 10.05 | |
| S1 | 37.55 | 0.09 | 0.10 | 0.26 | 58.50 | 0.71 | 2.80×10-3 | 1.00×10-4 | 3.30×10-3 | ||||||
| L2 | 30.43 | 21.24 | 48.33 | 1.220 | 6.78 | 89.88 | 17.04 | 19.57 | 0.56 | 200.00 | 14.28 | 0.38 | 18.00 | 30.16 | |
| S2 | 35.62 | 0.17 | 0.20 | 0.35 | 57.31 | 1.00 | 1.30×10-2 | 3.00×10-4 | 4.00×10-3 | ||||||
| L3 | 15.44 | 25.51 | 59.05 | 1.246 | 6.40 | 55.56 | 32.08 | 38.60 | 0.28 | 207.48 | 24.80 | 0.69 | 33.04 | 50.08 | |
| S3 | 36.82 | 0.30 | 0.36 | 0.27 | 56.99 | 0.90 | 8.30×10-3 | 3.00×10-4 | 4.20×10-3 | ||||||
| L4 | 10.76 | 26.80 | 62.44 | 1.266 | 5.96 | 34.56 | 37.40 | 54.64 | 0.23 | 222.60 | 33.28 | 0.98 | 46.84 | 80.24 | |
| S4 | 31.70 | 5.26 | 0.62 | 0.15 | 54.81 | 1.31 | 1.30×10-2 | 5.00×10-4 | 3.10×10-3 | ||||||
| L5 | 7.50 | 27.84 | 64.66 | 1.287 | 5.33 | 16.40 | 24.44 | 76.50 | 0.14 | 236.16 | 45.12 | 1.38 | 65.24 | 100.13 | |
| S5 | 20.17 | 17.00 | 1.34 | 0.12 | 50.28 | 1.43 | 2.40×10-2 | 1.10×10-3 | 5.90×10-3 | ||||||
| L6 | 4.33 | 29.28 | 66.39 | 1.312 | 4.05 | 6.56 | 10.00 | 95.00 | 7.20×10-2 | 264.44 | 42.44 | 2.15 | 105.20 | 178.51 | |
| S6 | 5.71 | 8.12 | 8.53 | 4.70×10-2 | 34.64 | 8.70 | 4.20×10-2 | 1.50×10-3 | 1.20×10-2 | ||||||
| L7 | 1.71 | 30.71 | 67.58 | 1.360 | 3.65 | 1.72 | 0.76 | 126.08 | 5.00×10-2 | 338.32 | 43.40 | 4.44 | 216.68 | 312.72 | |
| S7 | 1.63 | 2.72 | 12.65 | 1.50×10-2 | 36.40 | 7.04 | 0.12 | 5.70×10-3 | 1.70×10-2 | ||||||
| [1] | 张苏江,张琳,姜爱玲,等.中国盐湖资源开发利用现状与发展建议[J].无机盐工业,2022,54(10):13-21. |
| ZHANG Sujiang, ZHANG Lin, JIANG Ailing,et al.Current situation and development suggestions of development and utilization of salt lake resources in China[J].Inorganic Chemicals Industry,2022,54(10):13-21. | |
| [2] | 王淑丽,祁才吉,崔博京,等.中国钾盐资源勘查趋势分析与展望[J].地质学报,2024,98(10):2978-2988. |
| WANG Shuli, QI Caiji, CUI Bojing,et al.Analysis and prospect of exploration trends of potassium salt resources in China[J].Acta Geologica Sinica,2024,98(10):2978-2988. | |
| [3] | 伊跃军,张秀峰,张利珍,等.中国钾盐资源开发利用现状及建议[J].无机盐工业,2024,56(10):12-19. |
| YI Yuejun, ZHANG Xiufeng, ZHANG Lizhen,et al.Present situation and suggestions on exploitation and utilization of potassium salt resources in China[J].Inorganic Chemicals Industry,2024,56(10):12-19. | |
| [4] | 乜贞,伍倩,丁涛,等.中国盐湖卤水提锂产业化技术研究进展[J].无机盐工业,2022,54(10):1-12. |
| NIE Zhen, WU Qian, DING Tao,et al.Research progress on industrialization technology of lithium extraction from salt lake brine in China[J].Inorganic Chemicals Industry,2022,54(10):1-12. | |
| [5] | 马珍.盐湖锂资源高效分离提取技术研究进展[J].无机盐工业,2022,54(10):22-29. |
| MA Zhen.Research progress on efficient separation and extraction technology of lithium resources in salt lakes[J].Inorganic Chemicals Industry,2022,54(10):22-29. | |
| [6] | 付煜,邓觅,黄冬根,等.盐湖卤水提锂技术研究进展[J].无机盐工业,2023,55(9):9-16,65. |
| FU Yu, DENG Mi, HUANG Donggen,et al.Research progress of lithium extraction technology from salt lake brine[J].Inorganic Chemicals Industry,2023,55(9):9-16,65. | |
| [7] | 王丹,蒋宗胜,田忠华,等.硼的地球化学特征及富集成矿机制[J].岩石学报,2024,40(10):3238-3256. |
| WANG Dan, JIANG Zongsheng, TIAN Zhonghua,et al.Boron geochemical characteristics and the mechanisms of enrichment and mineralization[J].Acta Petrologica Sinica,2024,40(10):3238-3256. | |
| [8] | 蔚昊学,李庆宽,都永生,等.青藏高原盐湖卤水中溴的分布特征及来源初探[J].湖泊科学,2024,36(3):827-835. |
| YU Haoxue, LI Qingkuan, DU Yongsheng,et al.Distribution characteristics and possible sources of bromine in salt lakes on the Qinghai-Xizang Plateau[J].Journal of Lake Sciences,2024,36(3):827-835. | |
| [9] | 宣之强.青海昆特依和马海盐湖区钾镁盐矿床固体矿的基本特征[J].盐湖研究,1995,3(4):1-9. |
| XUAN Zhiqiang.Basic characteristic of potassium and magnesium solid deposit in Kunteyi and Mahai salt lake of Qinghai Province[J].Journal of Salt Lake Research,1995,3(4):1-9. | |
| [10] | 崔庆岗,赵淑芳.柴达木盆地马海盐湖的盐壳特征及其地质意义[J].化工矿产地质,2018,40(4):218-226. |
| CUI Qinggang, ZHAO Shufang.The characteristics and geological significance of ring-shaped salt crustin Mahai salt lake of Qaidam Basin in Qinghai Province[J].Geology of Chemical Minerals,2018,40(4):218-226. | |
| [11] | 龙鹏宇,赵艳军,胡宇飞,等.马海盐湖北部矿段低品位固体钾矿中钾盐矿物的赋存特征及成因探讨[J].地球学报,2022,43(3):338-346. |
| LONG Pengyu, ZHAO Yanjun, HU Yufei,et al.Occurrence characteristics and genetic study of potassium salt minerals in low grade solid potassium ore in the north section of Mahai saltlake[J].Acta Geoscientica Sinica,2022,43(3):338-346. | |
| [12] | 赵全升,孔智涵,胡舒娅,等.柴达木盆地马海盐湖地下卤水地球物理探测及应用[J].吉林大学学报(地球科学版),2023,53(5):1560-1572. |
| ZHAO Quansheng, KONG Zhihan, HU Shuya,et al.Geophysical exploration and application of underground brine of Mahai salt lake in Qaidam Basin[J].Journal of Jilin University(Earth Science Edition),2023,53(5):1560-1572. | |
| [13] | 赵英杰,胡舒娅,赵全升,等.开采条件下马海盐湖地下卤水水化学演化特征[J].世界地质,2020,39(3):693-699. |
| ZHAO Yingjie, HU Shuya, ZHAO Quansheng,et al.Hydrochemical evolution characteristics of underground brine under mining conditions in Mahai salt lake[J].Global Geology,2020,39(3):693-699. | |
| [14] | 边红利,呼桂桂.马海盐湖浮选法生产氯化钾排放尾盐中钾资源的强制浸取技术研究[J].盐湖研究,2014,22(1):32-36,60. |
| BIAN Hongli, HU Guigui.Technology research of mechanical agitation leaching potassium resources of flotation tailings from production of potassium chloride of Mahai saline lake[J].Journal of Salt Lake Research,2014,22(1):32-36,60. | |
| [15] | 李海民,何继辉,胡生忠.马海盐湖钾矿区北部矿段低品位地表固体钾矿开发利用主要方法[J].盐湖研究,2021,29(3):1-8. |
| LI Haimin, HE Jihui, HU Shengzhong.Main methods of development and utilization of low grade surface solid potassium ore in the north section of Mahai salt lake potassium mining area[J].Journal of Salt Lake Research,2021,29(3):1-8. | |
| [16] | 李洪普,郑绵平,侯献华,等.柴达木黑北凹地早更新世新型砂砾层卤水水化学特征与成因[J].地球化学(中国地质大学学报),2014,39(10):1333-1342. |
| LI Hongpu, ZHENG Mianping, HOU Xianhua,et al.Hydrochemistry characteristics and origin of new brine sandy gravel in early pleistocene of Heibei concave in Qaidam Basin[J].Earth Science-Journal of China University of Geosciences,2014,39(10):1333-1342. | |
| [17] | 李洪普,郑绵平,侯献华,等.柴达木西部南翼山构造富钾深层卤水矿的控制因素及水化学特征[J].地球学报,2015,36(1):41-50. |
| LI Hongpu, ZHENG Mianping, HOU Xianhua,et al.Control factors and water chemical characteristics of potassium-rich deep brine in Nanyishan structure of western Qaidam Basin[J].Acta Geoscientica Sinica,2015,36(1):41-50. | |
| [18] | 焦鹏程,张建伟,姚佛军,等.马海盐湖深部卤水钾盐勘查与研究进展[J].矿床地质,2016,35(6):1305-1308. |
| JIAO Pengcheng, ZHANG Jianwei, YAO Fojun,et al.Exploration and research progress of potassium salt in deep brine of Mahai Salt Lake[J].Mineral Deposits,2016,35(6):1305-1308. | |
| [19] | 岳鑫,刘溪溪,路亮,等.马海盆地深部孔隙卤水矿床水化学特征及成因[J].沉积学报,2019,37(3):532-540. |
| YUE Xin, LIU Xixi, LU Liang,et al.Hydrochemical characteristics and origin of deep pore brine deposits in Mahai Basin[J].Acta Sedimentologica Sinica,2019,37(3):532-540. | |
| [20] | 邹松,方霖,沈善强,等.国内外典型硫酸盐型盐湖卤水资源现状及提钾工艺综述[J].矿产保护与利用,2017,37(5):113- 118. |
| ZOU Song, FANG Lin, SHEN Shanqiang,et al.Resources situation and process research on potassium extraction in typical sulphate-type salt lakes at home and abroad[J].Conservation and Utilization of Mineral Resources,2017,37(5):113-118. | |
| [21] | 时历杰,王敏.柴达木盆地一里坪盐湖卤水水化学及夏季蒸发中钾、锂、硼行为[J].湖泊科学,2019,31(2):590-608. |
| SHI Lijie, WANG Min.Hydrochemistry and behavior of K,Li and B in summer evaporation of Yiliping salt lake brine in Qaidam Basin[J].Journal of Lake Sciences,2019,31(2):590-608. | |
| [22] | 苏建军,曾英,李陇岗,等.西台吉乃尔盐湖析钾老卤及冻卤25 ℃等温蒸发实验研究[J].无机盐工业,2022,54(5):84-89,95. |
| SU Jianjun, ZENG Ying, LI Longgang,et al.Study on isothermal evaporation of potassium-extracted old brine and frozen brine in West Taijinar Salt Lake at 25 ℃[J].Inorganic Chemicals Industry,2022,54(5):84-89,95. | |
| [23] | 王冀洺,乜贞,樊馥,等.西台吉乃尔盐湖盐田与模拟蒸发过程卤水钾、锂的损失研究[J].无机盐工业,2023,55(5):31- 38. |
| WANG Jiming, NIE Zhen, FAN Fu,et al.Study on potassium and lithium resource loss in brine of West Taijinar Salt Lake and simulated evaporation process[J].Inorganic Chemicals Industry,2023,55(5):31-38. | |
| [24] | 杨游胜,姚智豪,赵志星,等.富锂硫酸盐型盐湖卤水蒸发实验研究进展[J].无机盐工业,2024,56(4):1-7. |
| YANG Yousheng, YAO Zhihao, ZHAO Zhixing,et al.Research progress of lithium-rich sulfate type salt lake brine evaporation experiment[J].Inorganic Chemicals Industry,2024,56(4):1-7. | |
| [25] | 雷延智,潘玉麟.巴仑马海盐湖低品位卤水自然蒸发试验研究[J].化工矿物与加工,2006,35(6):11-14. |
| LEI Yanzhi, PAN Yulin.Natural evaporation of low grade brine in Balunmahai Salt Lake[J].Industrial Minerals & Processing,2006,35(6):11-14. | |
| [26] | 郭爱武,李刚.马海盐湖卤水自然蒸发实验研究[J].盐湖研究,2008,16(3):30-32. |
| GUO Aiwu, LI Gang.Study on natural evaporation of brine in Mahai salt lakes[J].Journal of Salt Lake Research,2008,16(3):30-32. | |
| [27] | 潘晓晨,成怀刚,程芳琴.马海盐湖溶采卤水高温梯级蒸发实验研究[J].无机盐工业,2013,45(6):15-18. |
| PAN Xiaochen, CHENG Huaigang, CHENG Fangqin.Study on stepped high temperatrue evaporation of dissolved brine in Mahai salt lakes[J].Inorganic Chemicals Industry,2013,45(6):15-18. | |
| [28] | 李建国,关云山,戴杰,等.马海盐湖低品位钾矿溶采卤水蒸发过程相图分析及计算[J].无机盐工业,2013,45(12):17-20. |
| LI Jianguo, GUAN Yunshan, DAI Jie,et al.Phase diagram analysis and computation for the process of brine evaporation of dissolution mining of low grade solid potassium deposit in Mahai salt lake[J].Inorganic Chemicals Industry,2013,45(12):17-20. | |
| [29] | 地矿部岩石矿物分析编写组.岩石矿物分析第二分册[M].4版.北京:地质出版社,2011. |
| [30] | 梁震.潜江凹陷广华地区锂卤水水化学特征与成因分析[D].武汉:长江大学,2024. |
| LIANG Zhen.Analysis on hydrochemical characteristics and origin of lithium brine in Guanghua area of Qianjiang depression[D].Wuhan:Yangtze University,2024. | |
| [31] | 金作美,肖显志,梁式梅.(Na+、K+、Mg2+),(Cl-、SO4 2-),H2O五元系统介稳平衡的研究[J].化学学报,1980,38(4):313-321. |
| JIN Zuomei, XIAO Xianzhi, LIANG Shimei.Study of the metastable equilibrium for pentanary system of(Na+,K+,Mg2+),(Cl-,SO4 2-),H2O[J].Acta Chimica Sinica,1980,38(4):313-321. | |
| [32] | 唐元晖,柏元吉,郭强,等.高矿化度矿井水热法脱盐过程中硫酸钙的结垢趋势预测及验证[J].化工学报,2025,76(1):81-92,2. |
| TANG Yuanhui, BAI Yuanji, GUO Qiang,et al.Prediction and verification of calcium sulfate scaling trend in hydrothermal desalination process of high salinity mine[J].CIESC Journal,2025,76(1):81-92,2. | |
| [33] | 时历杰,王敏.一里坪硫酸镁亚型盐湖钾镁混盐转化-浮选中物相行为[J].化工学报,2019,70(5):1832-1841. |
| SHI Lijie, WANG Min.Phase behavior of K-Mg mixed salt during transformation-flotation from Yiliping magnesium sulfate-type salt lake[J].CIESC Journal,2019,70(5):1832-1841. |
| [1] | YU Xudong, LI Jing, REN Siying, LUO Jun, ZENG Ying. Study on solid-liquid phase equilibrium of Li+,K+,Ca2+//Cl--H2O quaternary system at 298.2 K [J]. Inorganic Chemicals Industry, 2025, 57(3): 30-35. |
| [2] | LAI Xianrong, CHEN Zhouqin, SUN Hao, YANG Chao. Pilot study on lithium extraction by adsorption from raw brine of magnesium sulfate subtype salt lakes in Tibet [J]. Inorganic Chemicals Industry, 2023, 55(11): 86-92. |
| [3] | WU Liping,YUAN Hongzhan,JIN Fang. Study on natural evaporation experimental of deep brine in Yahu structure [J]. Inorganic Chemicals Industry, 2022, 54(11): 71-78. |
| [4] | JIN Fang,LI Hongpu,CHANG Donghai. Experiment on natural evaporation of brine in Nanyishan anticline structural area of Qaidam Basin [J]. Inorganic Chemicals Industry, 2021, 53(11): 86-90. |
| [5] | Han Guang,Han Jibin,Liu Jiubo,Hou Xianhua,Chen Jinniu,Cao Yizhang. Variation characteristics of LiCl deposit under condition of mining in East Taijnar Salt Lake,Qaidam Basin [J]. Inorganic Chemicals Industry, 2020, 52(12): 17-22. |
| [6] | BI Si-Feng, CUI Xiang-Mei. Freezing conversion of brine in Yiliping salt lake [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(9): 26-. |
| [7] | XIE Shao-Lei, ZHANG Chao, JI 律, CHEN Gao-Qi, JING Yan. Research on freezing crystalization behavior of magnesium sulfate subtypes brine at low temperature [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(2): 35-. |
| [8] | TAN Xiu-Min, ZHANG Xiu-Feng, ZHANG Li-Zhen. Study on bromine extraction technology of Jiangling depression deep brine [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(5): 13-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||