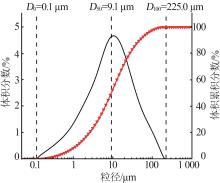
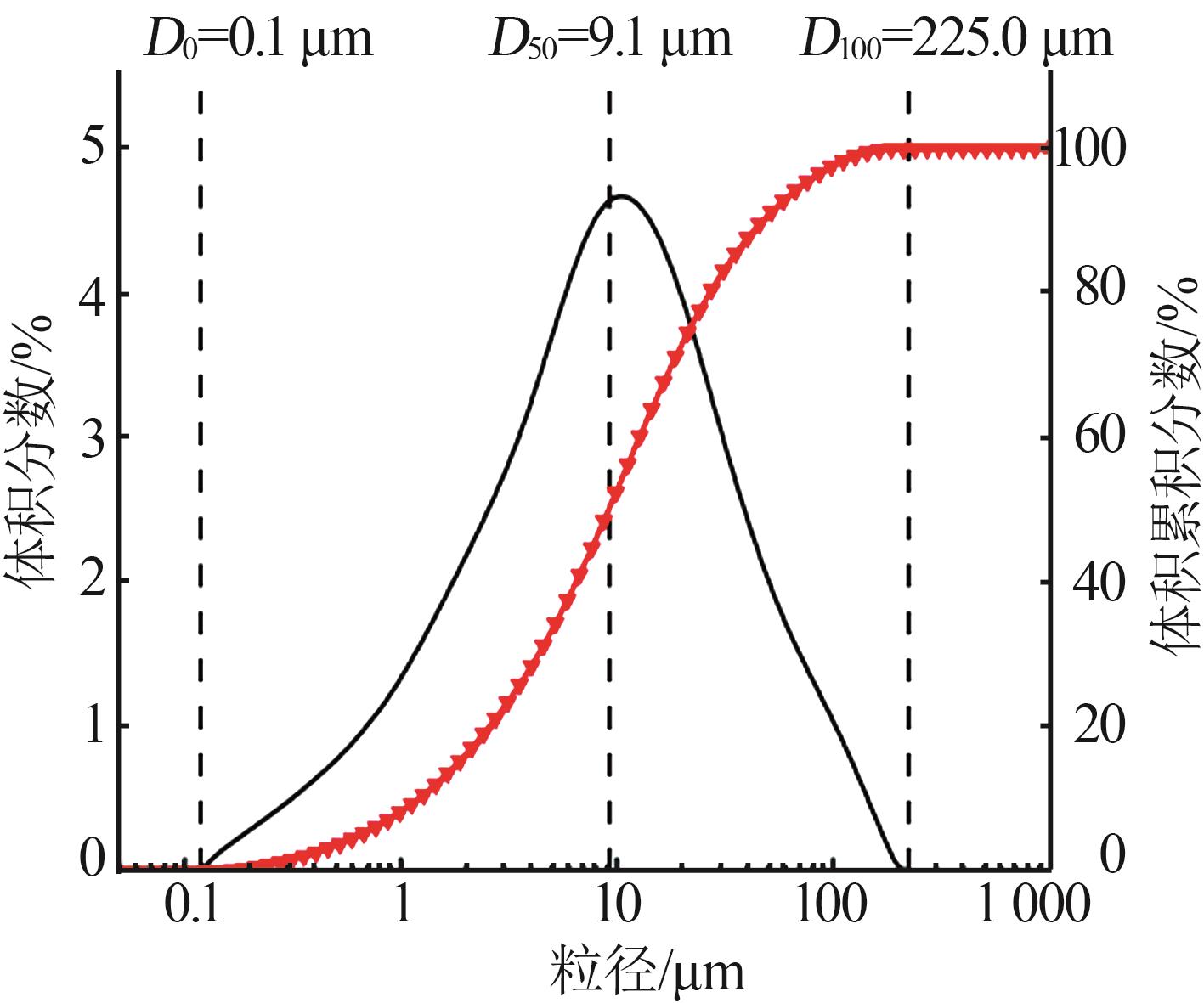
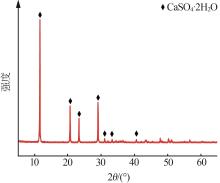
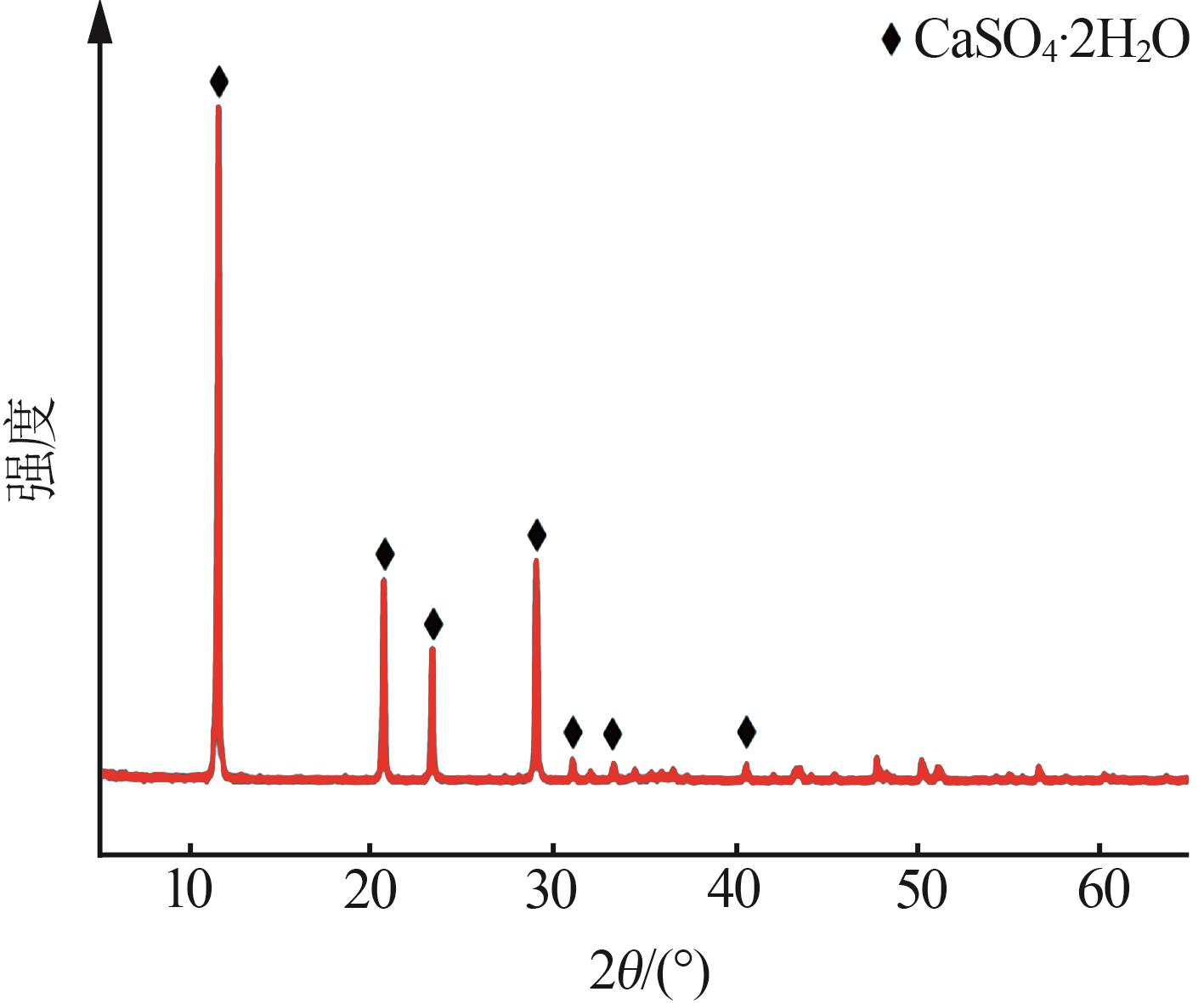
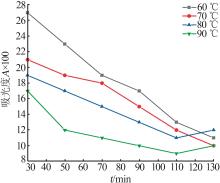
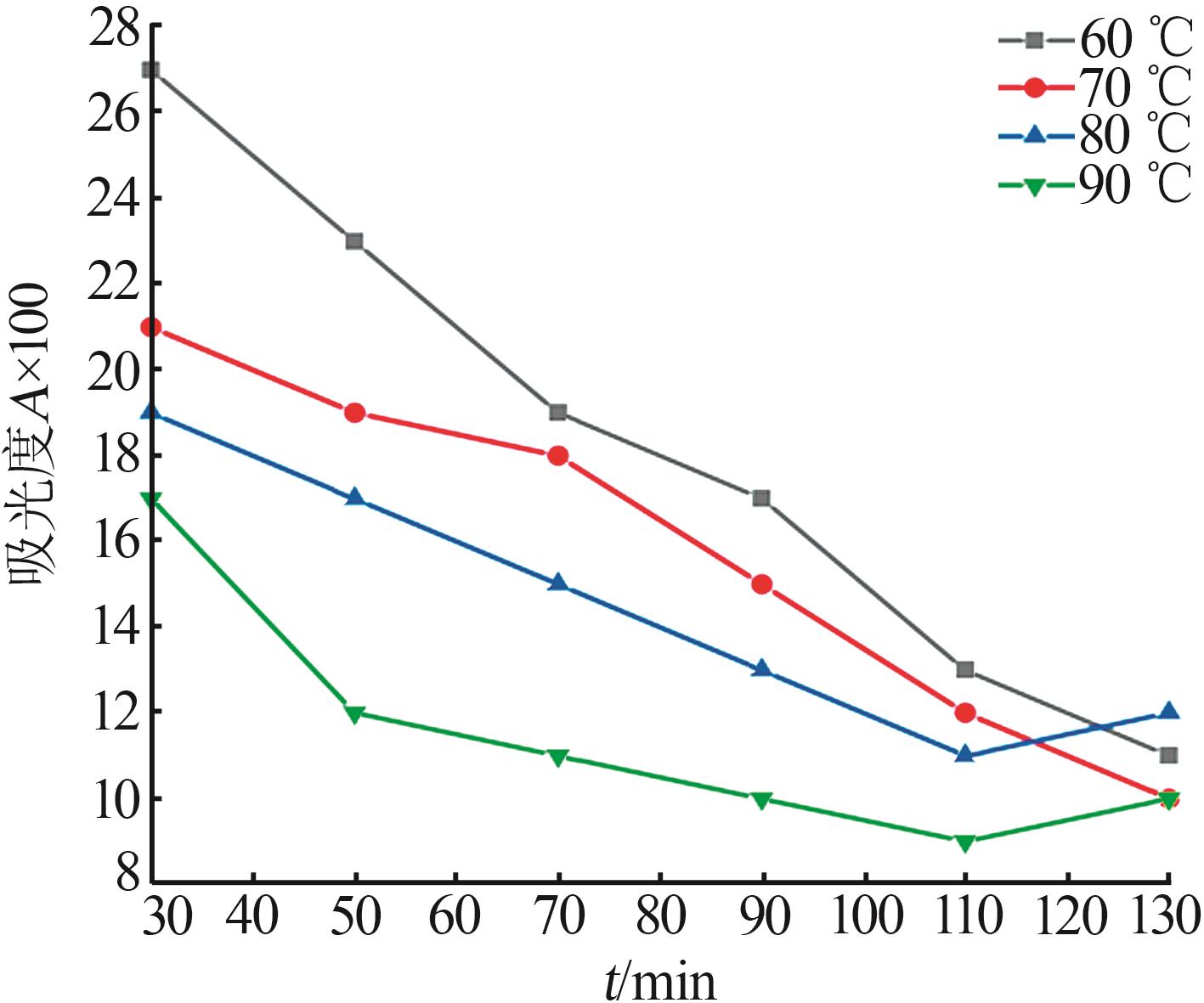
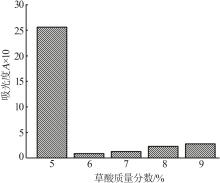
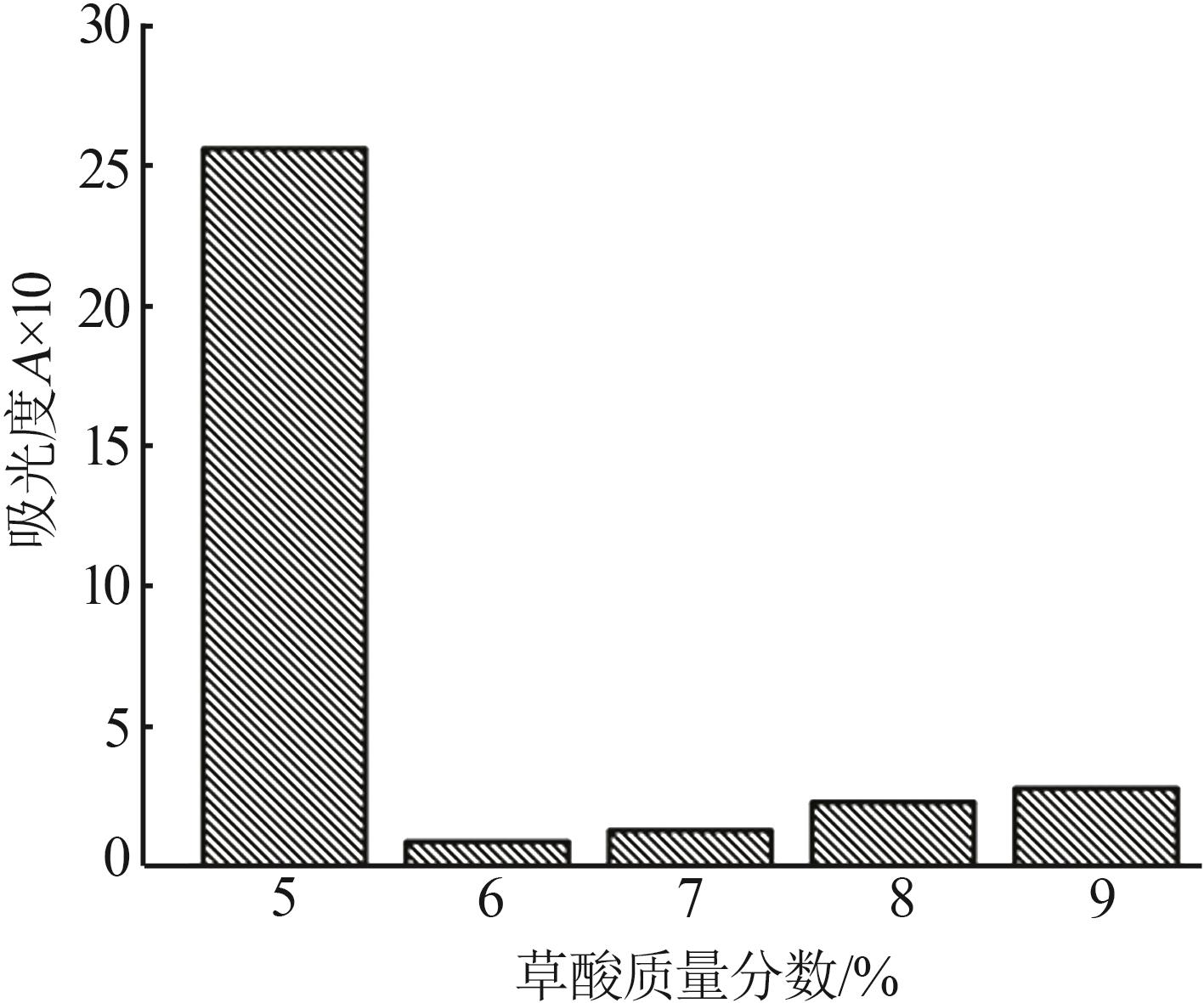
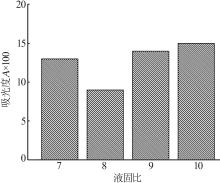
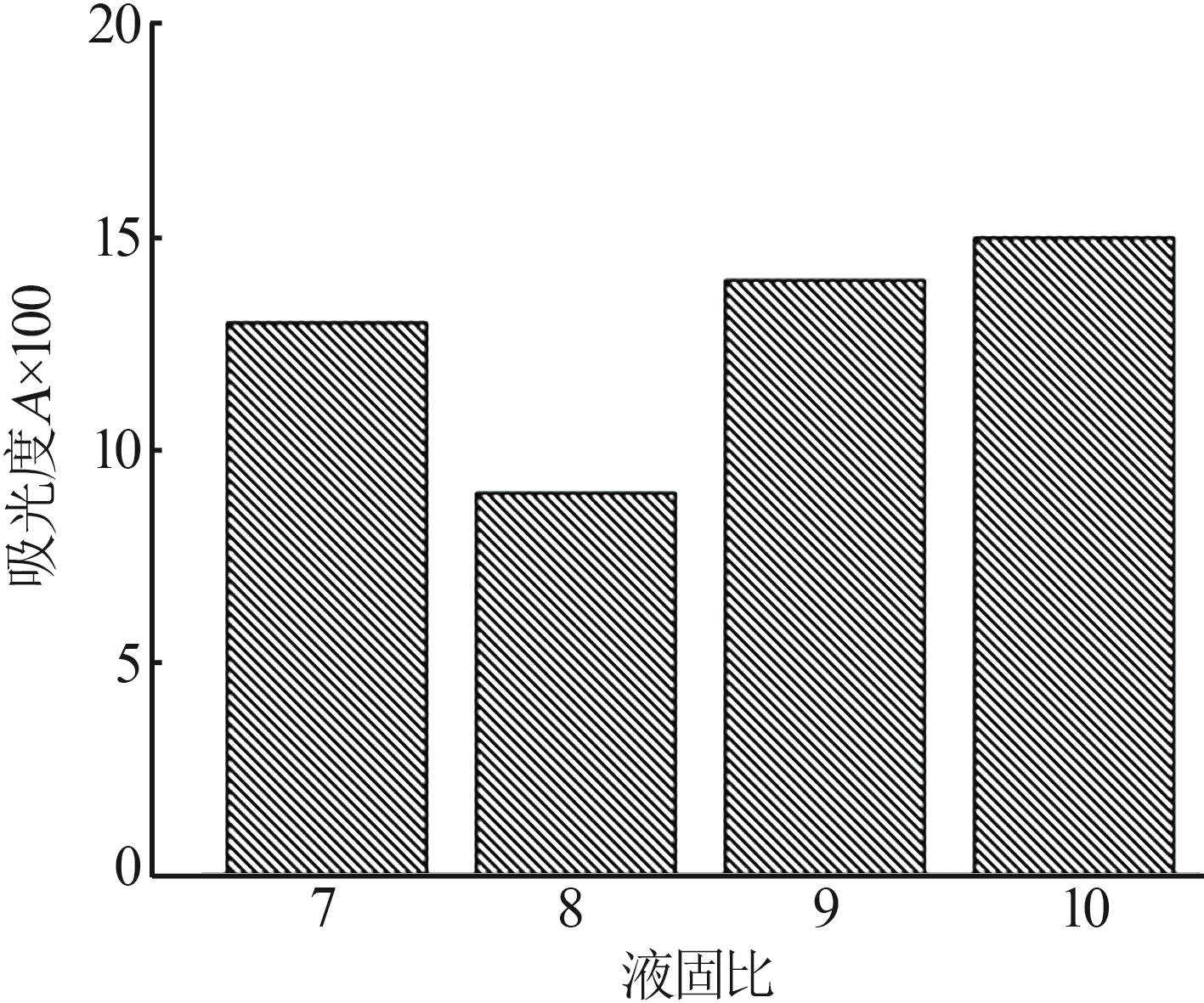
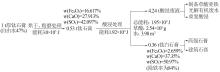
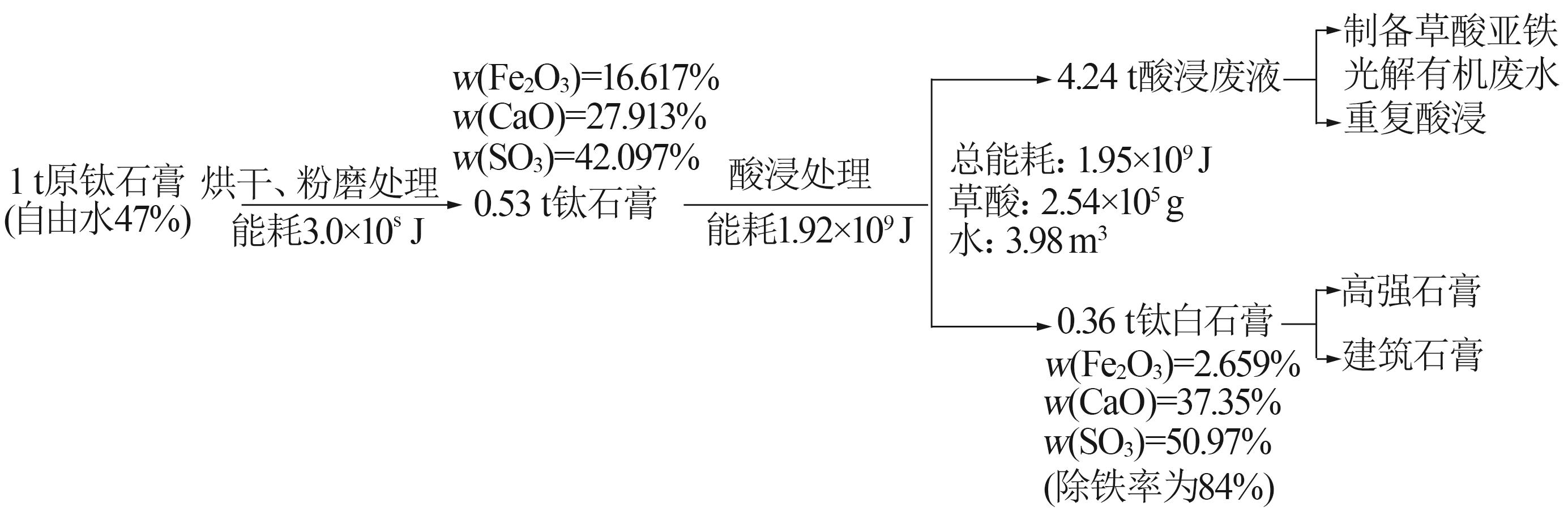
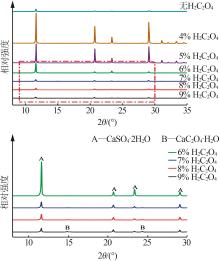
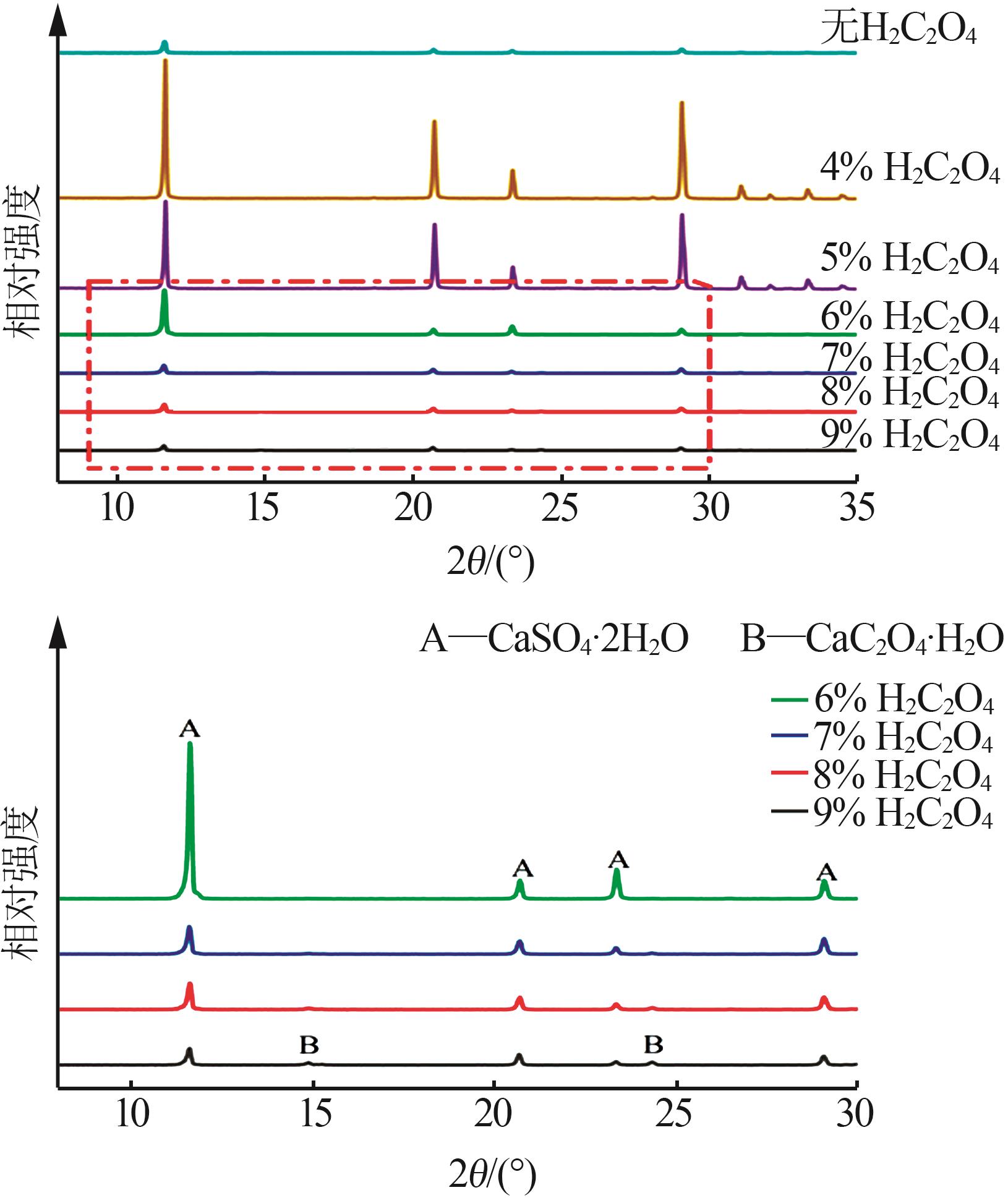
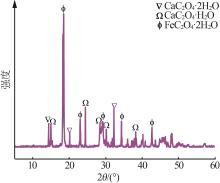
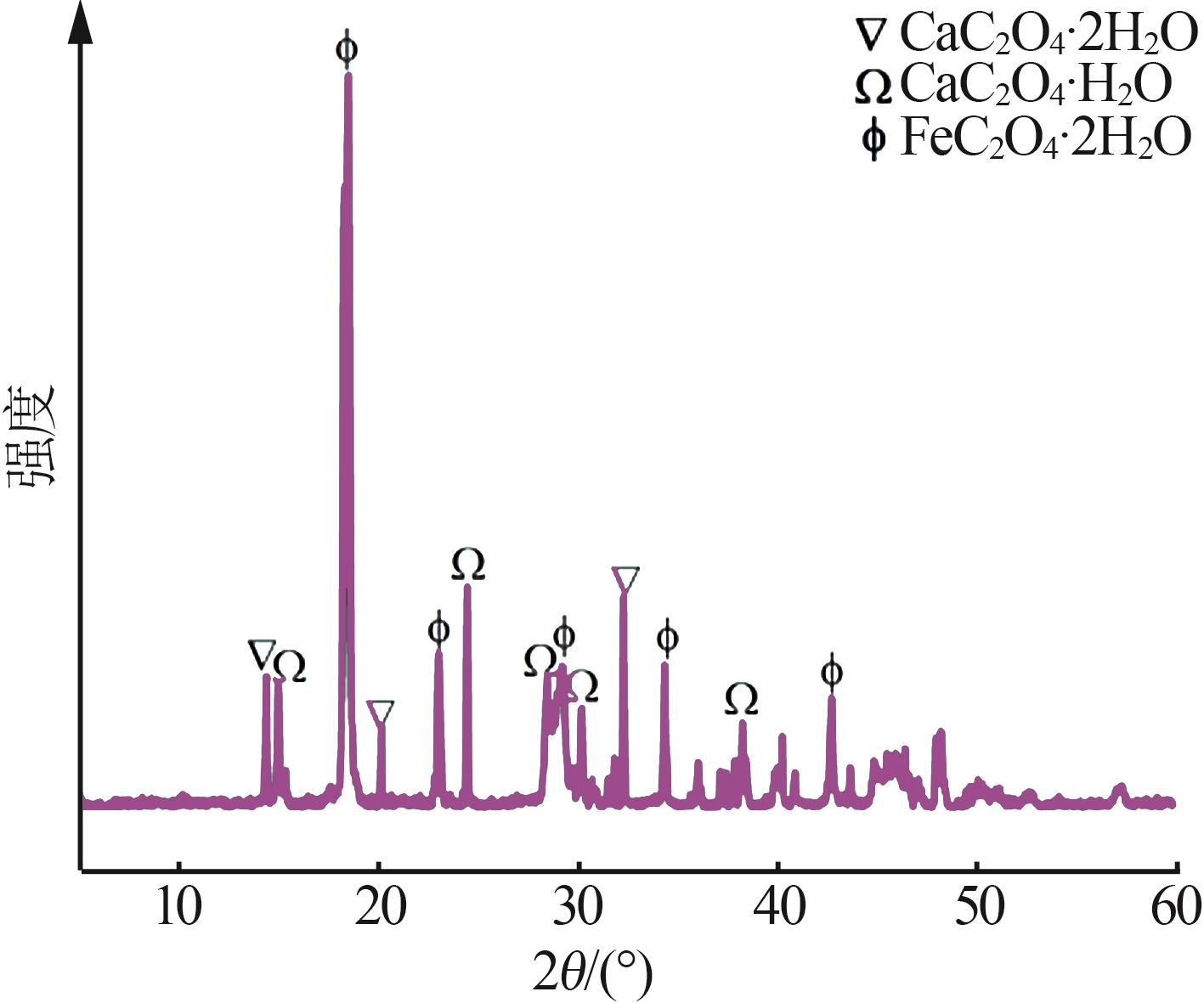
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (5): 108-114.doi: 10.19964/j.issn.1006-4990.2023-0345
• Environment·Health·Safety • Previous Articles Next Articles
Study on leaching treatment of titanium gypsum with oxalic acid
WANG Luwei( ), WANG Jie, LI Keke, FENG Chunhua(
), WANG Jie, LI Keke, FENG Chunhua( ), ZHANG Wenyan, ZHU Jianping
), ZHANG Wenyan, ZHU Jianping
- College of Material Science and Engineering,Henan Polytechnic University,Jiaozuo 454002,China
-
Received:2023-06-30Online:2024-05-10Published:2024-05-15 -
Contact:FENG Chunhua E-mail:2475245798@qq.com;fengchunhua@hpu.edu.cn
CLC Number:
Cite this article
WANG Luwei, WANG Jie, LI Keke, FENG Chunhua, ZHANG Wenyan, ZHU Jianping. Study on leaching treatment of titanium gypsum with oxalic acid[J]. Inorganic Chemicals Industry, 2024, 56(5): 108-114.
share this article
| 1 | 龚家竹.钛石膏与磷石膏固废耦合资源化利用技术进展[J].无机盐工业,2019,51(1):1-6,11. |
| GONG Jiazhu.Progress in coupling utilization technology of titanium gypsum and phosphogypsum solid waste[J].Inorganic Che-Industry micals,2019,51(1):1-6,11. | |
| 2 | YANG Yuhong, KOU Lidong, CHEN Huan,et al.Synthesis of magnetic adsorbents from titanium gypsum and biomass wastes for enhanced phosphate removal[J].Bioresource Technology,2023,371:128609. |
| 3 | 杜传伟,李国忠,陈娟.利用天然石膏形态组成模拟钛石膏及其性能研究[J].建筑材料学报,2014,17(3):511-516. |
| DU Chuanwei, LI Guozhong, CHEN Juan.Simulation of titanium gypsum with morphology and composition of natural gypsum and its property[J].Journal of Building Materials,2014,17(3):511-516. | |
| 4 | PENG Xiaoqian, ZHENG Jiayi, LIU Qian,et al.Efficient removal of iron from red gypsum via synergistic regulation of gypsum phase transformation and iron speciation[J].The Science of the Total Environment,2021,791:148319. |
| 5 | 顾汉念,郭腾飞,马时成,等.赤泥中铁的提取与回收利用研究进展[J].化工进展,2018,37(9):3599-3608. |
| GU Hannian, GUO Tengfei, MA Shicheng,et al.Review on separation,recovery,extraction and comprehensive utilization of iron from red mud[J].Chemical Industry and Engineering Progress,2018,37(9):3599-3608. | |
| 6 | LI Xiong, ZHU Rong, HU Shuxuan,et al.Leaching of arsenic from flue gas desulphurisation gypsum using ultrasound-enhanced sulfuric acid[J].Journal of Cleaner Production,2022,381:135163. |
| 7 | 王倩,彭同江,孙红娟,等.钛石膏-H2SO4-H2O体系中石膏-硬石膏的相变规律[J].硅酸盐通报,2022,41(2):725-732. |
| WANG Qian, PENG Tongjiang, SUN Hongjuan,et al.Phase transformation law of gypsum-anhydrite in titanium gypsum-H2SO4-H2O system[J].Bulletin of the Chinese Ceramic Society,2022,41(2):725-732. | |
| 8 | 赵雪淞,刘鑫,李彩霞,等.混酸提纯制备高纯石英砂及浸出动力学分析[J].硅酸盐学报,2021,49(3):581-589. |
| ZHAO Xuesong, LIU Xin, LI Caixia,et al.Preparation of high-purity quartz sand by leaching with mixed acids[J].Journal of the Chinese Ceramic Society,2021,49(3):581-589. | |
| 9 | SULTANA U K, KURNY A S W.Dissolution kinetics of iron oxides in clay in oxalic acid solutions[J].International Journal of Minerals,Metallurgy,and Materials,2012,19(12):1083-1087. |
| 10 | 陈书锐,杨绍利,马兰,等.盐酸浸出钛石膏实验研究[J].无机盐工业,2020,52(2):65-68. |
| CHEN Shurui, YANG Shaoli, MA Lan,et al.Study on leaching of titanium gypsum with hydrochloric acid[J].Inorganic Chemicals Industry,2020,52(2):65-68. | |
| 11 | YU Zhanglong, SHI Zhixia, CHEN Yongmei,et al.Red-mud treatment using oxalic acid by UV irradiation assistance[J].Transactions of Nonferrous Metals Society of China,2012,22(2):456- 460. |
| 12 | 石志霞,余章龙,彭程,等.低品位铝土矿草酸光催化除铁研究[J].材料导报,2015,29(S2):125-128. |
| SHI Zhixia, YU Zhanglong, PENG Cheng,et al.Sunlight irradiation-assisted removal of iron oxide from low grade bauxite using oxalic acid[J].Materials Reports,2015,29(S2):125-128. | |
| 13 | 党晓娥,张婷.Fe3+-C2O4 2--H2O溶液中Fe(Ⅲ)的形态分布及其在硫酸烧渣和氰化尾渣除铁过程中的应用[J].化工进展,2023(11):6005-6014. |
| DANG Xiao′e, ZHANG Ting.Species distribution of Fe(Ⅲ)in Fe3+-C2O4 2--H2O solution and application in the process of iron removal from sulphuric acid cinder and syanide tailings[J].Chemical Industry and Engineering Progress,2023(11):6005-6014. | |
| 14 | 彭家惠,张建新,瞿金东,等.有机酸对α半水脱硫石膏晶体生长习性的影响与调晶机理[J].硅酸盐学报,2011,39(10):1711-1718. |
| PENG Jiahui, ZHANG Jianxin, QU Jindong,et al.Effect of organic acid on crystalline habit of α-hemihydrate desulfurization gypsum and its crystal modification mechanism[J].Journal of the Chinese Ceramic Society,2011,39(10):1711-1718. | |
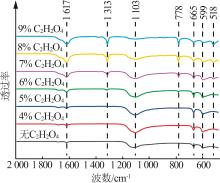
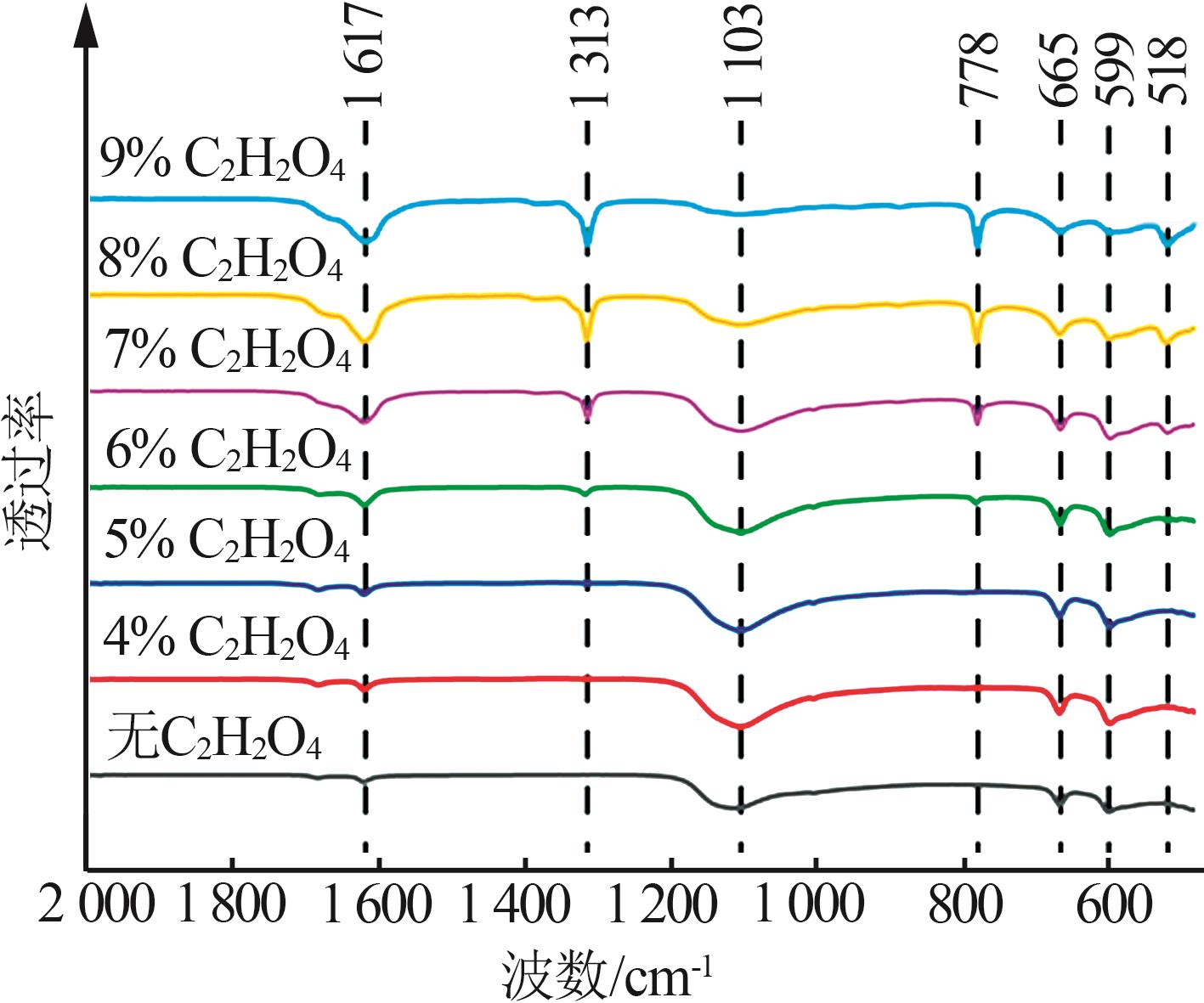
| 15 | 陈岚,满瑞林,李志明.草酸钙结石形态的红外光谱分析[J].分析测试学报,2007,26(4):582-584. |
| CHEN Lan, MAN Ruilin, LI Zhiming.Analysis of calcium oxalate crystalline phases by infrared spectrophotometry[J].Journal of Instrumental Analysis,2007,26(4):582-584. | |
| 16 | 闫蔚,曾柏淋,孟江,等.石膏红外图谱鉴定研究[J].光谱学与光谱分析,2016,36(7):2098-2103. |
| YAN Wei, ZENG Bolin, MENG Jiang,et al.Study on the identification of gypsum fibrosum with FTIR[J].Spectroscopy and Spectral Analysis,2016,36(7):2098-2103. | |
| 17 | ZHANG Wenyan, ZHAO Liya, XUE Mengfen,et al.Effect of oxalic acid pretreatment on the mechanical properties and microstructure of phosphogypsum[J].Construction and Building Materials,2023,362:129631. |
| [1] | XIANG Linghui, WU Lin, LIU Ying, HU Ya. Study on application of phosphogypsum oxalic acid pretreatment liquid in oriented silicon steel coatings [J]. Inorganic Chemicals Industry, 2024, 56(10): 103-109. |
| [2] | XIANG Mengqi, MENG Hua, WANG Ye, MENG Xianzhang, BAI Yuhang, WANG Yujunyao, ZHANG Yidan. Study on kinetic of iron leaching from titanium gypsum and its cyclic acid leaching process [J]. Inorganic Chemicals Industry, 2024, 56(1): 114-120. |
| [3] | LI Shuqi, LI Xinqian, FENG Haitao, LI Bo, NIU Zhengrong, DONG Yaping. Study on separation of iron from chromium trichloride solution [J]. Inorganic Chemicals Industry, 2023, 55(8): 91-94. |
| [4] | WEI Kang, ZHANG Hao, GAN Shunpeng. Research on mechanical drive enhancement crystallization process for titanium gypsum [J]. Inorganic Chemicals Industry, 2023, 55(11): 78-85. |
| [5] | MA Lei,SHENG Yu,ZHOU Junhong,YANG Yujun,LUO Hui,PAN Chunying,WANG Guo. Study on new process of comprehensive utilization and separation of sulfur and calcium of titanium gypsum [J]. Inorganic Chemicals Industry, 2022, 54(7): 124-128. |
| [6] | WANG Youyou,YUAN Hao,HAN Qingqing,CHEN Shiying. Activation of activator on fly ash?titanium gypsum?calcium carbide slag system and its hydration mechanism [J]. Inorganic Chemicals Industry, 2022, 54(6): 115-119. |
| [7] | Meng Hua,Zhao Jingyi,Nie Chaoyang,Wang Ye. Study on simulation process of reducing titanium dioxide gypsum by sulfur fluidization [J]. Inorganic Chemicals Industry, 2021, 53(9): 61-66. |
| [8] | Lu Bo,Liu Yiqiang,Liu Xin,Jiang Wei,Wu Xi. Study on preparation of ultrafine lanthanum oxide powder by precipitation with oxalic acid [J]. Inorganic Chemicals Industry, 2021, 53(5): 66-68. |
| [9] | Shao Wujun,Liu Chunyan,He Ning,Guo Hongchen. Effect of oxalic acid solution treatment on physiochemical properties and catalytic performance of SAPO-34 molecular sieve [J]. Inorganic Chemicals Industry, 2020, 52(2): 84-90. |
| [10] | Chen Shurui,Yang Shaoli,Ma Lan,Hou Jing. Study on leaching of titanium gypsum with hydrochloric acid [J]. Inorganic Chemicals Industry, 2020, 52(2): 65-68. |
| [11] | Shi Congyun,Wang Jinfeng,Liu Weijie,Cai Benzhe,Zhao Runsheng,Liang Yangshuo,Yan Xiaochao,Jiang Yufan. Mechanism of oxalic acid effect on enhancing the oil absorptionvalue of optical biber preform waste powder [J]. Inorganic Chemicals Industry, 2019, 51(7): 61-63. |
| [12] | YANG Cheng. Experimental study on barium titanate preparation method [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(8): 44-. |
| [13] | WU Yan-Ni, ZUO Bai-Yan, LI Ling, ZONG Jun. Preparation of nano-sized magnesia with industrial oxalic acid and magnesium hydroxide [J]. INORGANICCHEMICALSINDUSTRY, 2011, 43(7): 29-. |
| [14] | ZHANG Yue, YUAN Li, LIU Jin-Ping, LI Xiao-Lei, ZHAO Hong, SONG Xiao-Li. Solid phase synthesis of LiBOB for lithium batteries [J]. INORGANICCHEMICALSINDUSTRY, 2011, 43(4): 42-. |
| [15] | Cheng Zhongjian;Chang Yupu;Chen Yingjun. Production of electronic-grade high purity barium titanate [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(9): 0-0. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||