Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (3): 7-17.doi: 10.19964/j.issn.1006-4990.2021-0628
• Reviews and Special Topics • Previous Articles Next Articles
Study on controllable synthesis and ultramicroscopic structure-activity relationship of cerium oxides nanocatalysts
HAO Xiaodong1( ),FENG Xinyi1,2,XU Yang1,2,ZHANG Xishu1,2,LIU Wen3,HAO Fangyuan3,MA Shufang1,XU Bingshe1
),FENG Xinyi1,2,XU Yang1,2,ZHANG Xishu1,2,LIU Wen3,HAO Fangyuan3,MA Shufang1,XU Bingshe1
- 1. Materials Institute of Atomic and Molecular Science,Shaanxi University of Science & Technology,Xi′An 710021,China
2. School of Materials Science and Engineering,Shaanxi University of Science & Technology
3. Shaanxi Normal University
-
Received:2021-11-10Online:2022-03-10Published:2022-03-18
CLC Number:
Cite this article
HAO Xiaodong,FENG Xinyi,XU Yang,ZHANG Xishu,LIU Wen,HAO Fangyuan,MA Shufang,XU Bingshe. Study on controllable synthesis and ultramicroscopic structure-activity relationship of cerium oxides nanocatalysts[J]. Inorganic Chemicals Industry, 2022, 54(3): 7-17.
share this article

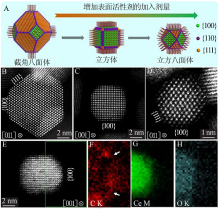
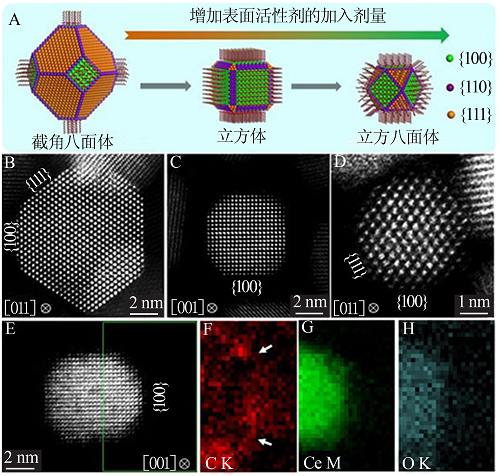
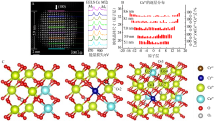
Fig.3
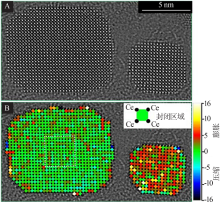
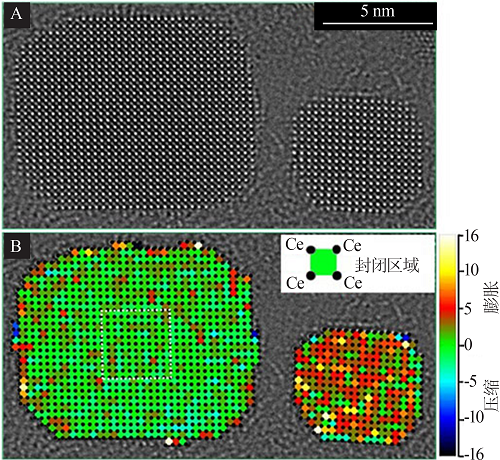
Direct observation and analysis of the surfactant at the surface of CeO2 nanocrystals:Elemental EELS mapping of the distribution of surfactant on the surface of CeO2 nanocrystals(A);Optimized STEM imaging condition for a single molecular chain of organic surfactant on CeO2 nanocrystals(B)"

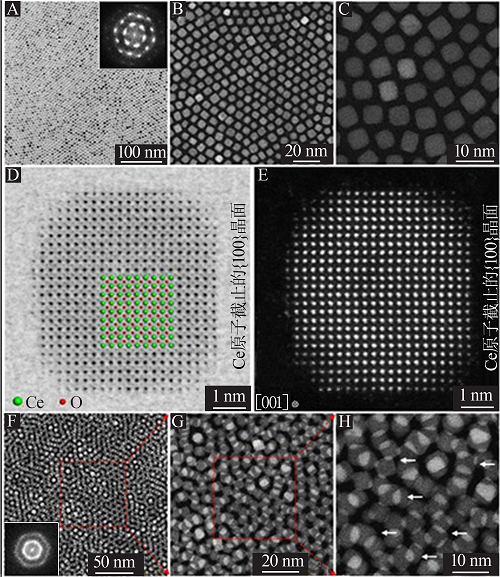
Fig.4
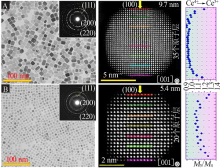
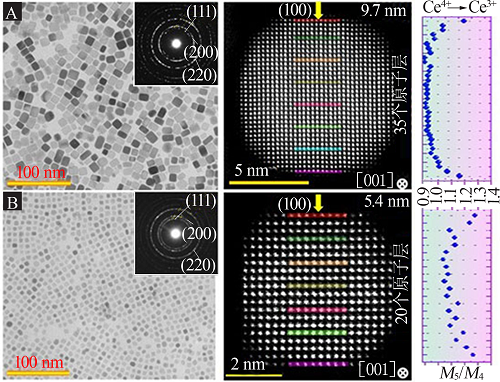
Self-assembly of CeO2 nanocrystals:Low magnification TEM and HAADF-STEM images of monolayer cubic CeO2 NCs superstructure(A~C),the inset in A is the corresponding FFT pattern from the cubic CeO2 NCs superstructure;ABF-and HAADF-STEM images of a single cubic CeO2 nanocrystal(D~E) ; HAADF-STEM images of the double layer CeO2 NCs supers-tructure(F~H),the inset in F is the corresponding FFT pattern"
| [1] |
TROVARELLI A. Catalytic properties of ceria and CeO2-containing materials[J]. Catalysis Reviews, 1996, 38(4):439-520.
doi: 10.1080/01614949608006464 |
| [2] |
MURRAY E P, TSAI T, BARNETT S. A direct-methane fuel cell with a ceria-based anode[J]. Nature, 1999, 400(6745):649-651.
doi: 10.1038/23220 |
| [3] | XU C, QU X. Cerium oxide nanoparticle:A remarkably versatile rare earth nanomaterial for biological applications[J]. NPG Asia Materi-als, 2014, 6(3):102-108. |
| [4] |
ZHANG Y, ZHAO S, FENG J, et al. Unraveling the physical chemi-stry and materials science of CeO2-based nanostructures[J]. Chem, 2021, 7(8):2022-2059.
doi: 10.1016/j.chempr.2021.02.015 |
| [5] |
TAN Z, WU T S, SOO Y L, et al. Unravelling the true active site for CeO2-catalyzed dephosphorylation[J]. Applied Catalysis B:Environ-mental, 2020, 264.Doi: 10.1016/j.apcatb.2019.118508.
doi: 10.1016/j.apcatb.2019.118508 |
| [6] | 宋晓岚, 杨振华, 邱冠周, 等. 纳米氧化铈在高新技术领域中的应用及其制备研究进展[J]. 材料导报, 2003, 17(12):36-39. |
| [7] |
FENG X, LI W, LIU D, et al. Self-assembled Pd@CeO2/γ-Al2O3 ca-talysts with enhanced activity for catalytic methane combustion[J]. Small, 2017, 13(31).Doi: 10.1002/smll.201700941.
doi: 10.1002/smll.201700941 |
| [8] | LI L, ZHANG N, WU R, et al. Comparative study of moisture-treated Pd@CeO2/Al2O3 and Pd/CeO2/Al2O3 catalysts for automobile exhaust emission reactions:Effect of core-shell interface[J]. ACS Applied Materials & Interfaces, 2020, 12(9):10350-10358. |
| [9] |
MACHIDA M, FUJIWARA A, YOSHIDA H, et al. Deactivation me-chanism of Pd/CeO2-ZrO2 three-way catalysts analyzed by chassis-dynamometer tests and in situ diffuse reflectance spectroscopy[J]. ACS Catalysis, 2019, 9(7):6415-6424.
doi: 10.1021/acscatal.9b01669 |
| [10] |
FUJIWARA A, YOSHIDA H, OHYAMA J, et al. In-situ diffuse reflectance spectroscopy analysis of Pd/CeO2-ZrO2 model three-way catalysts under Lean-Rich cycling condition[J]. Catalysis Today, 2021, 376:269-275.
doi: 10.1016/j.cattod.2020.05.031 |
| [11] |
CHEN Y, FAN J, DENG J, et al. Synjournal of high stability nano-sized Rh/CeO2-ZrO2 three-way automotive catalysts by Rh chemi-cal state regulation[J]. Journal of the Energy Institute, 2020, 93(6):2325-2333.
doi: 10.1016/j.joei.2020.07.005 |
| [12] |
FAN J, CHEN Y, JIANG X, et al. A simple and effective method to synthesize Pt/CeO2 three-way catalysts with high activity and hy-drothermal stability[J]. Journal of Environmental Chemical En-gineering, 2020, 8(5).Doi: 10.1016/j.jece.2020.104236.
doi: 10.1016/j.jece.2020.104236 |
| [13] |
WANG Y G, MEI D, GLEZAKOU V A, et al. Dynamic formation of single-atom catalytic active sites on ceria-supported gold nanopar-ticles[J]. Nature Communications, 2015, 6.Doi: 10.1038/ncomms7511.
doi: 10.1038/ncomms7511 |
| [14] |
TAN Z, ZHANG J, CHEN Y C, et al. Unravelling the role of struc-tural geometry and chemical state of well-defined oxygen vacanci-es on pristine CeO2 for H2O2 activation[J]. The Journal of Physical Chemistry Letters, 2020, 11(14):5390-5396.
doi: 10.1021/acs.jpclett.0c01557 |
| [15] |
JIANG D, WAN G, GARCÍA-VARGAS C E, et al. Elucidation of the active sites in single-atom Pd1/CeO2 catalysts for low-tempera-ture CO oxidation[J]. ACS Catalysis, 2020, 10(19):11356-11364.
doi: 10.1021/acscatal.0c02480 |
| [16] |
TROVARELLI A, LLORCA J. Ceria catalysts at nanoscale:How do crystal shapes shape catalysis?[J]. ACS Catalysis, 2017, 7(7):4716-4735.
doi: 10.1021/acscatal.7b01246 |
| [17] |
ZHANG J, KUMAGAI H, YAMAMURA K, et al. Extra-low-tempe-rature oxygen storage capacity of CeO2 nanocrystals with cubic fa-cets[J]. Nano Letters, 2011, 11(2):361-364.
doi: 10.1021/nl102738n |
| [18] |
CARGNELLO M, DOAN-NGUYEN V V T, GORDON T R, et al. Control of metal nanocrystal size reveals metal-support interface role for ceria catalysts[J]. Science, 2013, 341(6147):771-773.
doi: 10.1126/science.1240148 |
| [19] |
DAO D V, NGUYEN T T D, UTHIRAKUMAR P, et al. Insightful understanding of hot-carrier generation and transfer in plasmonic Au@CeO2 core-shell photocatalysts for light-driven hydrogen evo-lution improvement[J]. Applied Catalysis B:Environmental, 2021, 286.Doi: 10.1016/j.apcatb.2021.119947.
doi: 10.1016/j.apcatb.2021.119947 |
| [20] |
LU Y, ZHOU S, KUO C T, et al. Unraveling the intermediate reac-tion complexes and critical role of support-derived oxygen atoms in CO oxidation on single-atom Pt/CeO2[J]. ACS Catalysis, 2021, 11(14):8701-8715.
doi: 10.1021/acscatal.1c01900 |
| [21] | MURAVEV V, SPEZZATI G, SU Y Q, et al. Interface dynamics of Pd-CeO2 single-atom catalysts during CO oxidation[J]. Nature Ca-talysis, 2021, 4(6):469-478. |
| [22] |
ZHANG L, SPEZZATI G, MURAVEV V, et al. Improved Pd/CeO2 catalysts for low-temperature NO reduction:Activation of CeO2 la-ttice oxygen by Fe doping[J]. ACS Catalysis, 2021, 11(9):5614-5627.
doi: 10.1021/acscatal.1c00564 |
| [23] |
TAN Z, LI G, CHOU H L, et al. Differentiating surface Ce species among CeO2 facets by solid-state NMR for catalytic correlation[J]. ACS Catalysis, 2020, 10(7):4003-4011.
doi: 10.1021/acscatal.0c00014 |
| [24] |
JIANG F, WANG S, LIU B, et al. Insights into the influence of CeO2 crystal facet on CO2 hydrogenation to methanol over Pd/CeO2 cata-lysts[J]. ACS Catalysis, 2020, 10(19):11493-11509.
doi: 10.1021/acscatal.0c03324 |
| [25] |
WEI Y, ZHANG Y, ZHANG P, et al. Boosting the removal of diesel soot particles by the optimal exposed crystal facet of CeO2 in Au/CeO2 catalysts[J]. Environmental Science & Technology, 2020, 54(3):2002-2011.
doi: 10.1021/acs.est.9b07013 |
| [26] |
LI C, SUN Y, DJERDJ I, et al. Shape-controlled CeO2 nanoparticl-es:Stability and activity in the catalyzed HCl oxidation reaction[J]. ACS Catalysis, 2017, 7(10):6453-6463.
doi: 10.1021/acscatal.7b01618 |
| [27] |
CORDEIRO M A L, WENG W, STROPPA D G, et al. High resolu-tion electron microscopy study of nanocubes and polyhedral nano-crystals of cerium(Ⅳ) oxide[J]. Chemistry of Materials, 2013, 25(10):2028-2034.
doi: 10.1021/cm304029s |
| [28] |
DANG F, KATO K, IMAI H, et al. Characteristics of CeO2 nanocu-bes and related polyhedra prepared by using a liquid-liquid inter-face[J]. Crystal Growth & Design, 2010, 10(10):4537-4541.
doi: 10.1021/cg1008347 |
| [29] |
ZHANG J, OHARA S, UMETSU M, et al. Colloidal ceria nanocry-stals:A tailor-made crystal morphology in supercritical water[J]. Advanced Materials, 2007, 19(2):203-206.
doi: 10.1002/(ISSN)1521-4095 |
| [30] |
HAO X, ZHANG S, XU Y, et al. Surfactant-mediated morphology evolution and self-assembly of cerium oxide nanocrystals for cata-lytic and supercapacitor applications[J]. Nanoscale, 2021, 13(23):10393-10401.
doi: 10.1039/D1NR01746B |
| [31] |
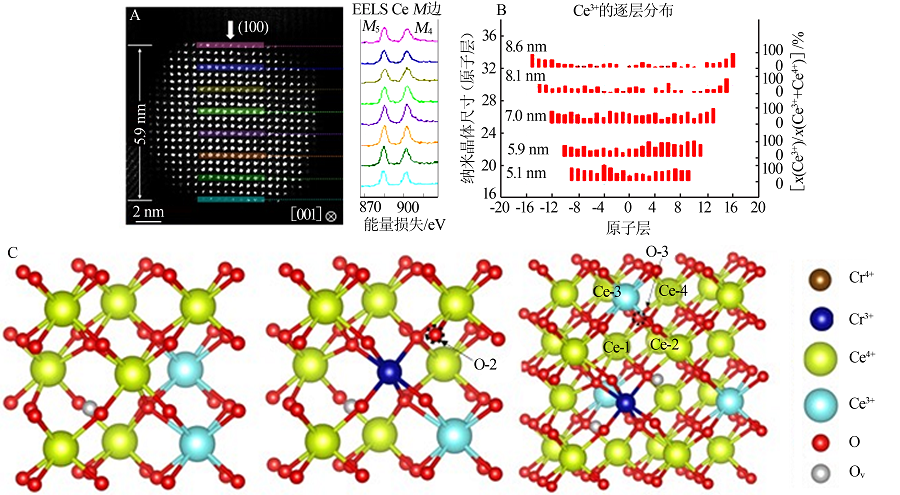
BOLES M A, ENGEL M, TALAPIN D V. Self-assembly of colloidal nanocrystals:From intricate structures to functional materials[J]. Chemical Reviews, 2016, 116(18):11220-11289.
doi: 10.1021/acs.chemrev.6b00196 |
| [32] | 孟令镕, 彭卿, 周和平, 等. 从纳米晶到三维超晶格结构[J]. 高等学校化学学报, 2011, 32(3):429-436. |
| [33] | YE X, CHEN J, ERIC IRRGANG M, et al. Quasicrystalline nano-crystal superlattice with partial matching rules[J]. Nature Materi-als, 2016, 16:214-219. |
| [34] |
SI K J, CHEN Y, SHI Q, et al. Nanoparticle superlattices:The roles of soft ligands[J]. Advanced Science, 2018, 5(1).Doi: 10.1002/advs.201700179.
doi: 10.1002/advs.201700179 |
| [35] |
NAGAOKA Y, ZHU H, EGGERT D, et al. Single-component qua-sicrystalline nanocrystal superlattices through flexible polygon tiling rule[J]. Science, 2018, 362(6421):1396-1400.
doi: 10.1126/science.aav0790 |
| [36] |
BRENNAN M C, TOSO S, PAVLOVETC I M, et al. Superlattices are greener on the other side:How light transforms self-assembled mixed halide perovskite nanocrystals[J]. ACS Energy Letters, 2020, 5(5):1465-1473.
doi: 10.1021/acsenergylett.0c00630 |
| [37] |
WU L, WILLIS J J, MCKAY I S, et al. High-temperature crystalliza-tion of nanocrystals into three-dimensional superlattices[J]. Nature, 2017, 548(7666):197-201.
doi: 10.1038/nature23308 |
| [38] |
WU L, MENDOZA-GARCIA A, LI Q, et al. Organic phase synthe-ses of magnetic nanoparticles and their applications[J]. Chemical Reviews, 2016, 116(18):10473-10512.
doi: 10.1021/acs.chemrev.5b00687 |
| [39] |
YANG Y, WANG B, SHEN X, et al. Scalable assembly of crystalli-ne binary nanocrystal superparticles and their enhanced magnetic and electrochemical properties[J]. Journal of the American Chemical Society, 2018, 140(44):15038-15047.
doi: 10.1021/jacs.8b09779 |
| [40] | 周学华, 李津如, 刘春艳, 等. 不同链长表面活性剂修饰的金纳米颗粒的制备、稳定性及二维排列[J]. 中国科学:B辑, 2002, 32(3):243-247. |
| [41] |
WEIDMAN M C, NGUYEN Q, SMILGIES D M, et al. Impact of size dispersity,ligand coverage,and ligand length on the structure of PbS nanocrystal superlattices[J]. Chemistry of Materials, 2018, 30(3):807-816.
doi: 10.1021/acs.chemmater.7b04322 |
| [42] | WINSLOW S W, SWAN J W, TISDALE W A. The importance of unbound ligand in nanocrystal superlattice formation[J]. Journal of the American Chemical Society, 2020, 142(21):9675-9685. |
| [43] |
OWEN J. The coordination chemistry of nanocrystal surfaces[J]. Science, 2015, 347(6222):615-616.
doi: 10.1126/science.1259924 |
| [44] |
BOLES M A, LING D, HYEON T, et al. The surface science of na-nocrystals[J]. Nature Materials, 2016, 15(2):141-153.
doi: 10.1038/nmat4526 |
| [45] |
YIN Y, ALIVISATOS A P. Colloidal nanocrystal synjournal and the organic-inorganic interface[J]. Nature, 2005, 437(7059):664-670.
doi: 10.1038/nature04165 |
| [46] |
LOVE J C, ESTROFF L A, KRIEBEL J K, et al. Self-assembled monolayers of thiolates on metals as a form of nanotechnology[J]. Chemical Reviews, 2005, 105(4):1103-1170.
doi: 10.1021/cr0300789 |
| [47] |
BOLES M A, TALAPIN D V. Connecting the dots[J]. Science, 2014, 344(6190):1340-1341.
doi: 10.1126/science.1256197 |
| [48] |
ANDERSON N C, HENDRICKS M P, CHOI J J, et al. Ligand ex-change and the stoichiometry of metal chalcogenide nanocrystals:Spectroscopic observation of facile metal-carboxylate displacement and binding[J]. Journal of the American Chemical Society, 2013, 135(49):18536-18548.
doi: 10.1021/ja4086758 |
| [49] |
HUTH F, GOVYADINOV A, AMARIE S, et al. Nano-FTIR abso-rption spectroscopy of molecular fingerprints at 20 nm spatial res-olution[J]. Nano Letters, 2012, 12(8):3973-3978.
doi: 10.1021/nl301159v |
| [50] |
SIMON P, BAHRIG L, BABURIN I A, et al. Interconnection of na-noparticles within 2D superlattices of PbS/oleic acid thin films[J]. Advanced Materials, 2014, 26(19):3042-3049.
doi: 10.1002/adma.201305667 |
| [51] |
FINDLAY S D, SHIBATA N, SAWADA H, et al. Robust atomic resolution imaging of light elements using scanning transmission electron microscopy[J]. Applied Physics Letters, 2009, 95(19). Doi: 10.1063/1.3265946.
doi: 10.1063/1.3265946 |
| [52] |
HARUTA M, YOSHIDA K, KURATA H, et al. Atomic resolution ADF-STEM imaging of organic molecular crystal of halogenated copper phthalocyanine[J]. Ultramicroscopy, 2008, 108(6):545-551.
doi: 10.1016/j.ultramic.2007.08.011 |
| [53] |
HAO X, CHEN C, SAITO M, et al. Direct imaging for single molec-ular chain of surfactant on CeO2 nanocrystals[J]. Small, 2018, 14(31).Doi: 10.1002/smll.201801093.
doi: 10.1002/smll.201801093 |
| [54] |
COROPCEANU I, BOLES M A, TALAPIN D V. Systematic mapp-ing of binary nanocrystal superlattices:The role of topology in phase selection[J]. Journal of the American Chemical Society, 2019, 141(14):5728-5740.
doi: 10.1021/jacs.8b12539 |
| [55] | YU C, GUO X, MUZZIO M, et al. Self-assembly of nanoparticles into two-dimensional arrays for catalytic applications[J]. Chemphy-schem, 2019, 20(1):23-30. |
| [56] |
FINDLAY S D, SHIBATA N, SAWADA H, et al. Dynamics of an-nular bright field imaging in scanning transmission electron microscopy[J]. Ultramicroscopy, 2010, 110(7):903-923.
doi: 10.1016/j.ultramic.2010.04.004 |
| [57] |
BONESCHANSCHER M P, EVERS W H, GEUCHIES J J, et al. Long-range orientation and atomic attachment of nanocrystals in 2D honeycomb superlattices[J]. Science, 2014, 344(6190):1377-1380.
doi: 10.1126/science.1252642 |
| [58] | LITWINOWICZ A A, TAKAMI S, ASAHINA S, et al. Formation dynamics of mesocrystals composed of organically modified CeO2 nanoparticles:Analogy to a particle formation model[J]. CrystEng Comm, 2019, 21(25):3836-3843. |
| [59] |
CAMPBELL C T, PEDEN C H. Oxygen vacancies and catalysis on ceria surfaces[J]. Science, 2005, 309(5735):713-714.
doi: 10.1126/science.1113955 |
| [60] |
SETVIN M, ASCHAUER U, SCHEIBER P, et al. Reaction of O2 with subsurface oxygen vacancies on TiO2 anatase(101)[J]. Sci-ence, 2013, 341(6149):988-991.
doi: 10.1126/science.1239879 |
| [61] |
GRACIANI J, MUDIYANSELAGE K, XU F, et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synjournal from CO2[J]. Science, 2014, 345(6196):546-550.
doi: 10.1126/science.1253057 |
| [62] |
WANG H, LUO S, ZHANG M, et al. Roles of oxygen vacancy and Ox- in oxidation reactions over CeO2 and Ag/CeO2 nanorod model catalysts[J]. Journal of Catalysis, 2018, 368:365-378.
doi: 10.1016/j.jcat.2018.10.018 |
| [63] | SINGH P, HEGDE M, GOPALAKRISHNAN J. Ce2/3Cr1/3O2+y:A new oxygen storage material based on the fluorite structure[J]. Chemi-stry of Materials, 2008, 20(23):7268-7273. |
| [64] |
LIU X, ZHOU K, WANG L, et al. Oxygen vacancy clusters promot-ing reducibility and activity of ceria nanorods[J]. Journal of the American Chemical Society, 2009, 131(9):3140-3141.
doi: 10.1021/ja808433d |
| [65] |
JIANG D, WANG W, ZHANG L, et al. Insights into the surface-de-fect dependence of photoreactivity over CeO2 nanocrystals with well-fefined crystal facets[J]. ACS Catalysis, 2015, 5(8):4851-4858.
doi: 10.1021/acscatal.5b01128 |
| [66] |
LI Y, WEI Z, GAO F, et al. Effect of oxygen defects on the catalytic performance of VOx /CeO2 catalysts for oxidative dehydrogenation of methanol[J]. ACS Catalysis, 2015, 5(5):3006-3012.
doi: 10.1021/cs502084g |
| [67] |
DEJHOSSEINI M, AIDA T, WATANABE M, et al. Catalytic crack-ing reaction of heavy oil in the presence of cerium oxide nanoparti-cles in supercritical water[J]. Energy & Fuels, 2013, 27(8):4624-4631.
doi: 10.1021/ef400855k |
| [68] |
SEONG G, YOKO A, INOUE R, et al. Selective chemical recovery from biomass under hydrothermal conditions using metal oxide na-nocatalyst[J]. The Journal of Supercritical Fluids, 2018, 133(2):726-737.
doi: 10.1016/j.supflu.2017.09.032 |
| [69] |
WU Z, LI M, HOWE J, et al. Probing defect sites on CeO2 nanocrys-tals with well-defined surface planes by Raman spectroscopy and O2 adsorption[J]. Langmuir, 2010, 26(21):16595-16606.
doi: 10.1021/la101723w |
| [70] |
WU L, WIESMANN H, MOODENBAUGH A R, et al. Oxidation state and lattice expansion of CeO2-x nanoparticles as a function of particle size[J]. Physical Review B, 2004, 69(12).Doi: 10.1103/PhysRevB.69.125415.
doi: 10.1103/PhysRevB.69.125415 |
| [71] |
TURNER S, LAZAR S, FREITAG B, et al. High resolution mapping of surface reduction in ceria nanoparticles[J]. Nanoscale, 2011, 3(8):3385-3390.
doi: 10.1039/c1nr10510h |
| [72] | PRIEUR D, BONANI W, POPA K, et al. Size dependence of lattice parameter and electronic structure in CeO2 nanoparticles[J]. Inor-ganic Chemistry, 2020, 59(8):5760-5767. |
| [73] |
TSUNEKAWA S, ISHIKAWA K, LI Z Q, et al. Origin of anomalous lattice expansion in oxide nanoparticles[J]. Physical Review Let-ters, 2000, 85(16).Doi: 10.1103/PhysRevLett.85.3440.
doi: 10.1103/PhysRevLett.85.3440 |
| [74] | ZHANG F, CHAN S W, SPANIER J E, et al. Cerium oxide nano-particles:Size-selective formation and structure analysis[J]. App-lied Physics Letters, 2002, 80(1):127-129. |
| [75] |
ZHOU X D, HUEBNER W. Size-induced lattice relaxation in CeO2 nanoparticles[J]. Applied Physics Letters, 2001, 79(21):3512-3514.
doi: 10.1063/1.1419235 |
| [76] |
DESHPANDE S, PATIL S, KUCHIBHATLA S V N T, et al. Size dependency variation in lattice parameter and valency states in na-nocrystalline cerium oxide[J]. Applied Physics Letters, 2005, 87(13).Doi: 10.1063/1.2061873.
doi: 10.1063/1.2061873 |
| [77] |
PEREBEINOS V, CHAN S W, ZHANG F. ‘Madelung model’pre-diction for dependence of lattice parameter on nanocrystal size[J]. Solid State Communications, 2002, 123(6):295-297.
doi: 10.1016/S0038-1098(02)00266-1 |
| [78] |
DIEHM P M, AGOSTON P, ALBE K. Size-dependent lattice expan-sion in nanoparticles:Reality or anomaly?[J]. ChemPhysChem, 2012, 13(10):2443-2454.
doi: 10.1002/cphc.201200257 |
| [79] |
HAO X, YOKO A, CHEN C, et al. Atomic-scale valence state distri-bution inside ultrafine CeO2 nanocubes and its size dependence[J]. Small, 2018, 14(42).Doi: 10.1002/smll.201802915.
doi: 10.1002/smll.201802915 |
| [80] |
KOBAYASHI S, INOUE K, KATO T, et al. Multiphase nanodomai-ns in a strained BaTiO3 film on a GdScO3 substrate[J]. Journal of Applied Physics, 2018, 123(6).Doi: 10.1063/1.5012545.
doi: 10.1063/1.5012545 |
| [81] |
ESCH F, FABRIS S, ZHOU L, et al. Electron localization deter-mines defect formation on ceria substrates[J]. Science, 2005, 309(5735):752-755.
doi: 10.1126/science.1111568 |
| [82] |
JERRATSCH J F, SHAO X, NILIUS N, et al. Electron localization in defective ceria films:A study with scanning-tunneling micros-copy and density-functional theory[J]. Physical Review Letters, 2011, 106(24).Doi: 10.1103/PhysRevLett.106.246801.
doi: 10.1103/PhysRevLett.106.246801 |
| [83] |
LIN Y, WU Z, WEN J, et al. Imaging the atomic surface structures of CeO2 nanoparticles[J]. Nano Letters, 2014, 14(1):191-196.
doi: 10.1021/nl403713b |
| [84] |
GILLISS S R, BENTLEY J, CARTER C B. Electron energy-loss spectroscopic study of the surface of ceria abrasives[J]. Applied Surface Science, 2005, 241(1/2):61-67.
doi: 10.1016/j.apsusc.2004.09.018 |
| [85] |
ZHANG F, WANG P, KOBERSTEIN J, et al. Cerium oxidation state in ceria nanoparticles studied with X-ray photoelectron spectro-scopy and absorption near edge spectroscopy[J]. Surface Science, 2004, 563(1/2/3):74-82.
doi: 10.1016/j.susc.2004.05.138 |
| [86] |
GORIS B, TURNER S, BALS S, et al. Three-dimensional valency mapping in ceria nanocrystals[J]. ACS Nano, 2014, 8(10):10878-10884.
doi: 10.1021/nn5047053 |
| [87] |
SUGIURA M. Oxygen storage materials for automotive catalysts: Ceria-zirconia solid solutions[J]. Catalysis Surveys from Asia, 2003, 7(1):77-87.
doi: 10.1023/A:1023488709527 |
| [88] |
DI MONTE R, KAŠPAR J. On the role of oxygen storage in three-way catalysis[J]. Topics in Catalysis, 2004, 28(1/2/3/4):47-57.
doi: 10.1023/B:TOCA.0000024333.08447.f7 |
| [89] |
SONG S H, MOON J, KIM J H, et al. Panoscopic alloying of cobalt in CeO2-ZrO2 solid solutions for superior oxygen-storage capaci-ty[J]. Acta Materialia, 2016, 113:206-212.
doi: 10.1016/j.actamat.2016.04.060 |
| [90] |
OZAWA M, MISAKI M, IWAKAWA M, et al. Low content Pt-doped CeO2 and core-shell type CeO2/ZrO2 model catalysts;microstruc-ture,TPR and three way catalytic activities[J]. Catalysis Today, 2019, 332:251-258.
doi: 10.1016/j.cattod.2018.08.015 |
| [91] |
YU J, WANG J, LONG X, et al. Formation of FeOOH nanosheets induces substitutional doping of CeO2-x with high-valence Ni for efficient water oxidation[J]. Advanced Energy Materials, 2021, 11(4).Doi: 10.1002/aenm.202002731.
doi: 10.1002/aenm.202002731 |
| [92] |
LIU B, LI C, ZHANG G, et al. Oxygen vacancy promoting dimethyl carbonate synjournal from CO2 and methanol over Zr-doped CeO2 nanorods[J]. ACS Catalysis, 2018, 8(11):10446-10456.
doi: 10.1021/acscatal.8b00415 |
| [93] |
KIM H J, SHIN D, JEONG H, et al. Design of an ultrastable and hi-ghly active ceria catalyst for CO oxidation by rare-earth-and tran-sition-metal Co-doping[J]. ACS Catalysis, 2020, 10(24):14877-14886.
doi: 10.1021/acscatal.0c03386 |
| [94] | YANG L, PASTOR-PÉREZ L, GU S, et al. Highly efficient Ni/CeO2-Al2O3 catalysts for CO2 upgrading via reverse water-gas shift:Effect of selected transition metal promoters[J]. Applied Catalysis B:En-vironmental, 2018, 232:464-471. |
| [95] |
SINGH P, HEGDE M. Ce0.67Cr0.33O2.11:A new low-temperature O2 ev-olution material and H2 generation catalyst by thermochemical splitting of water[J]. Chemistry of Materials, 2009, 22(3):762-768.
doi: 10.1021/cm9013305 |
| [96] |
ZHU Y, SEONG G, NOGUCHI T, et al. Highly Cr-substituted CeO2 nanoparticles synthesized using a non-equilibrium supercritical hydrothermal process:High oxygen storage capacity materials designed for a low-temperature bitumen upgrading process[J]. ACS Applied Energy Materials, 2020, 3(5):4305-4319.
doi: 10.1021/acsaem.0c00026 |
| [97] |
XIE H, WANG H, GENG Q, et al. Oxygen vacancies of Cr-doped CeO2 nanorods that efficiently enhance the performance of electro-catalytic N2 fixation to NH3 under ambient conditions[J]. Inorganic Chemistry, 2019, 58(9):5423-5427.
doi: 10.1021/acs.inorgchem.9b00622 |
| [98] | WANG Y, BAI X, WANG F, et al. Nanocasting synjournal of chro-mium doped mesoporous CeO2 with enhanced visible-light photo-catalytic CO2 reduction performance[J]. Journal of Hazardous Ma-terials, 2019, 372:69-76. |
| [99] |
LI X, WEI S, ZHANG Z, et al. Quantification of the active site den-sity and turnover frequency for soot combustion with O2 on Cr do-ped CeO2[J]. Catalysis Today, 2011, 175(1):112-116.
doi: 10.1016/j.cattod.2011.03.057 |
| [100] |
XU X, LIU L, TONG Y, et al. Facile Cr3+-doping strategy dramati-cally promoting Ru/CeO2 for low-temperature CO2 methanation: Unraveling the roles of surface oxygen vacancies and hydroxyl groups[J]. ACS Catalysis, 2021, 11(9):5762-5775.
doi: 10.1021/acscatal.0c05468 |
| [101] |
HAO X, YOKO A, INOUE K, et al. Atomistic origin of high-con-centration Ce3+ in {100}-faceted Cr-substituted CeO2 nanocryst-als[J]. Acta Materialia, 2021, 203.Doi: 10.1016/j.actamat.2020.11.015.
doi: 10.1016/j.actamat.2020.11.015 |
| [102] |
GANDUGLIA-PIROVANO M V, DA SILVA J L, SAUER J. Den-sity-functional calculations of the structure of near-surface oxygen vacancies and electron localization on CeO2{111}[J]. Physical Review Letters, 2009, 102(2).Doi: 10.1103/PhysRevLett.102.026101.
doi: 10.1103/PhysRevLett.102.026101 |
| [1] | LI Tong, YIN Hongfeng. Study on controllable preparation of nickel phyllosilicate nanotubes catalysts and their catalytic performance [J]. Inorganic Chemicals Industry, 2023, 55(5): 128-136. |
| [2] | JIA Yuhong, HU Zhongpan, WANG Kunyuan, HAN Jingfeng, WEI Yingxu, LIU Zhongmin. Co anchored on silanol nests of S-1 zeolite for propane dehydrogenation to propylene [J]. Inorganic Chemicals Industry, 2023, 55(5): 121-127. |
| [3] | YANG Wenbo,WU Pan,HE Jian,LIU Changjun,JIANG Wei. Study on effect of heating rate on structure-activity of g-C3N4 photocatalyst by pyrolysis of urea [J]. Inorganic Chemicals Industry, 2022, 54(4): 169-174. |
| [4] | DING Tian-Tian, FENG Li-Juan, YANG Wen-Chao, CHEN Xiao-Wei. Controllable synthesis and characterization of magnesium borate whisker [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(4): 45-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||