Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (12): 34-43.doi: 10.19964/j.issn.1000-4990.2022-0389
• Research & Development • Previous Articles Next Articles
Study on leaching and thermodynamic of spent lithium-ion batteries with electrochemical reduction
HE Ting1,2,3( ),KONG Jiao1,2,3,CUI Jingzhi4,CHEN Zhihao4,FU Tongtong4,GUO Zirui4,GU Shuai1,2,3(
),KONG Jiao1,2,3,CUI Jingzhi4,CHEN Zhihao4,FU Tongtong4,GUO Zirui4,GU Shuai1,2,3( ),YU Jianguo1,2,3(
),YU Jianguo1,2,3( )
)
- 1. National Engineering Research Center for Integrated Utilization of Salt Lake Resources,East China University of Science and Technology,Shanghai 200237,China
2. Engineering Research Center of Salt Lake Resources Process Engineering,Ministry of Education,East China University of Science and Technology
3. Joint International Laboratory for Potassium and Lithium Strategic Resources,East China University of Science and Technology
4. School of Chemical Engineering,East China University of Science and Technology
-
Received:2022-06-27Online:2022-12-10Published:2022-12-19 -
Contact:GU Shuai,YU Jianguo E-mail:y30210153@mail.ecust.edu.cn;gushuai@ecust.edu.cn;jgyu@ecust.edu.cn
CLC Number:
Cite this article
HE Ting,KONG Jiao,CUI Jingzhi,CHEN Zhihao,FU Tongtong,GUO Zirui,GU Shuai,YU Jianguo. Study on leaching and thermodynamic of spent lithium-ion batteries with electrochemical reduction[J]. Inorganic Chemicals Industry, 2022, 54(12): 34-43.
share this article
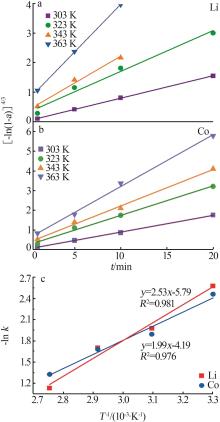
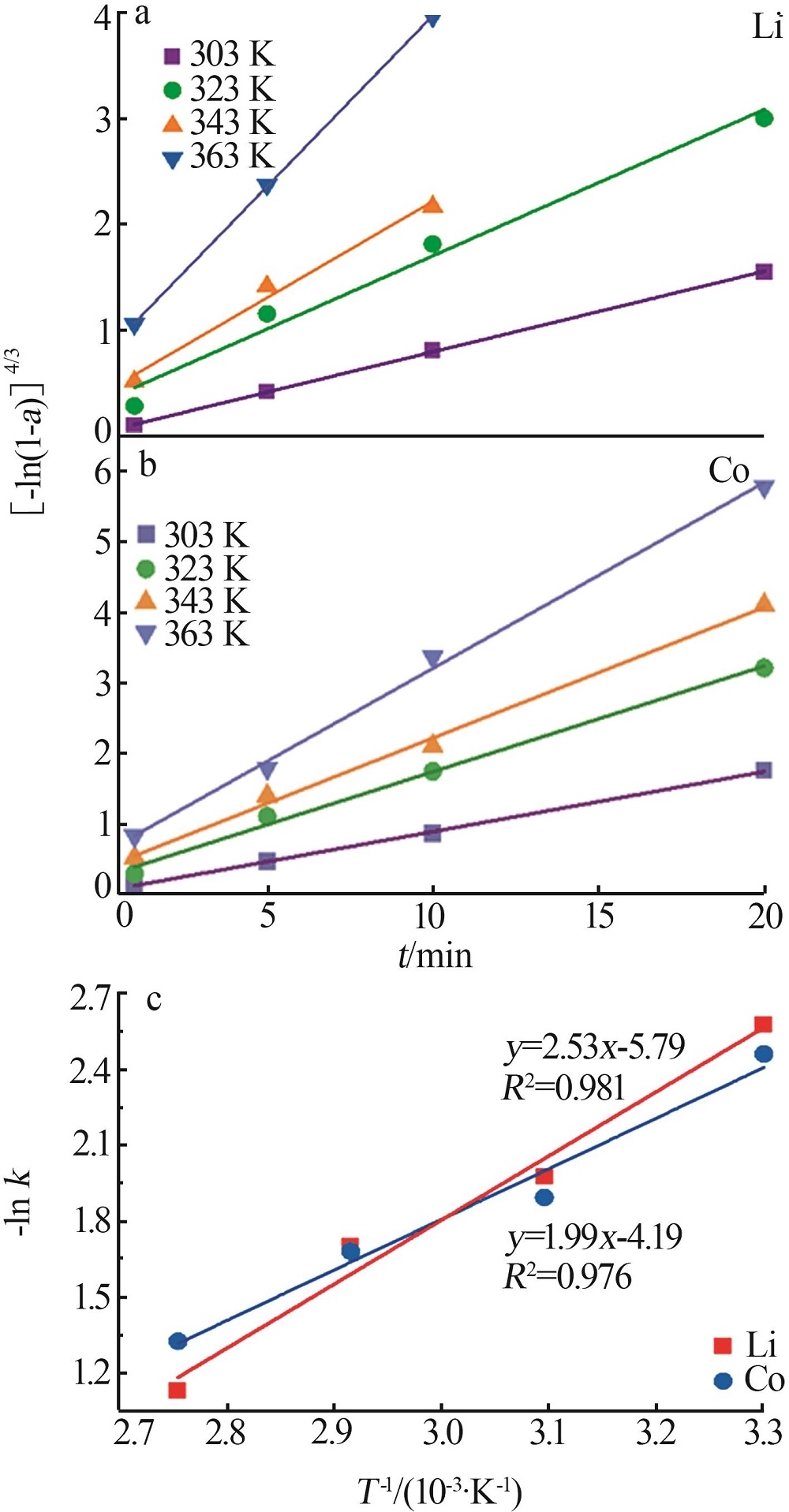
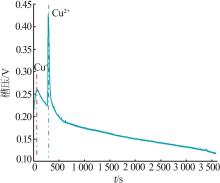
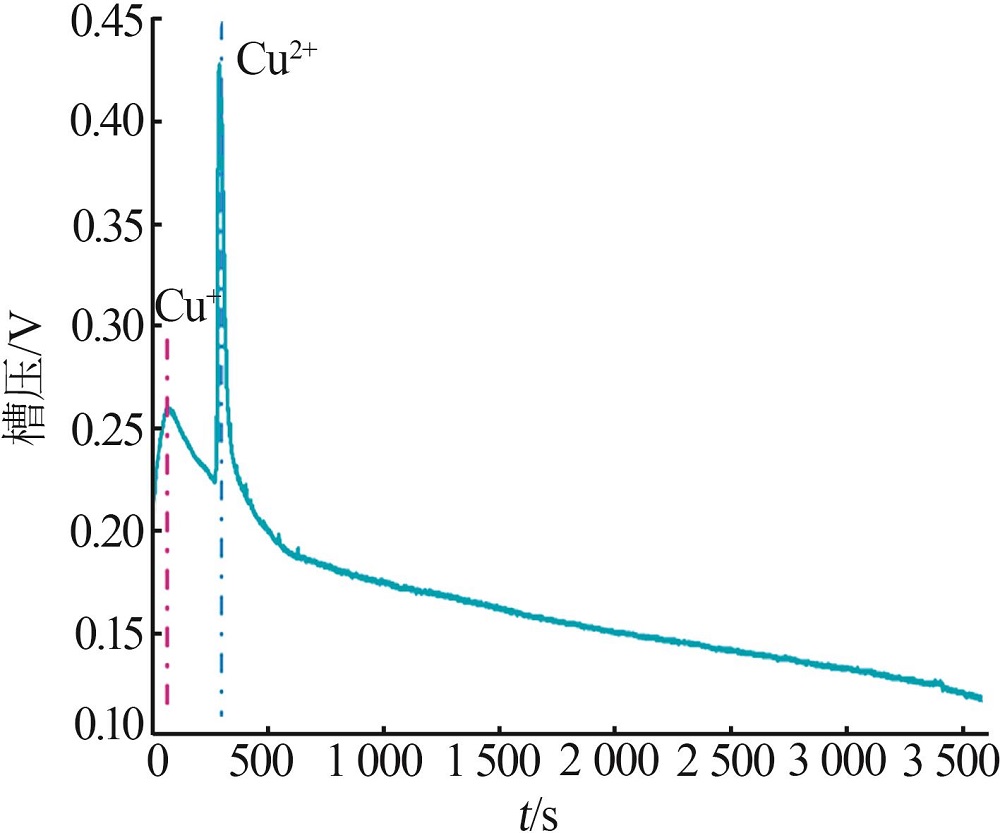
Table 1
Parameters of electrochemical recovery for spent LIBs cathode materials"
| 阴极材料 | 阳极材料 | 浸出机理 | 浸出率/% | 槽压/V |
|---|---|---|---|---|
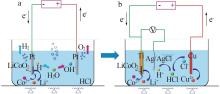
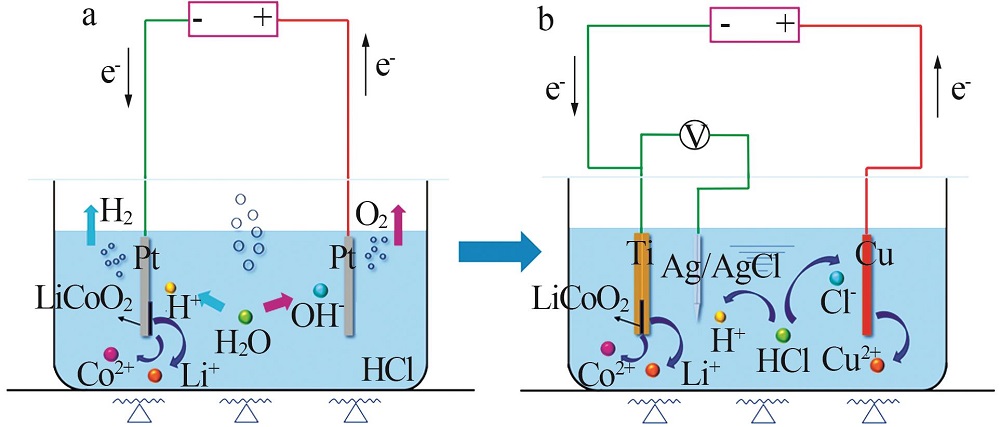
剥离铝箔,退役LiCoO2通 过导电胶粘接在Pt片上 | Pt片 | 阴极:LiCoO2+4H++e-=Li++Co2++2H2O 2H++2e-=H2 阳极:2H2O-4e-=4H++O2 | Li:94.2 Co:90.5 | 8[ |
| 带有铝箔的圆形退役LiCoO2 | Pt片 | 阴极:LiCoO2+4H++e-=Li++Co2++2H2O 2H++2e-=H2 阳极:2H2O-4e-=4H++O2 | Li:98.1 Co:97.4 | 8[ |
| 碳布(2 cm×5 cm) | LiNi x Co y Mn z O2(NCM) (2 cm×5 cm) | 阴极:4H++4e-=2H2 阳极:LiNi x Co y Mn z O2-εe-=εLi++Li1-ε Ni x Co y Mn z O2 4OH--4e-=O2+2H2O | Li:98.7 选择性:99.2 | 4[ |
| 钛网 | 退役LIBs正极材料 涂覆在碳纤维布上 | 阴极:δH++δe-=1/2δH2 δH2O=δH++δOH- 阳极:LiNi x Co y Mn z O2=δLi++Li1-δ Ni x Co y Mn z O2+δe- | Li:99.7 | 2[ |
| Pt片 | 退役LIBs正极材料 (2 cm×5 cm) | 阴极:4H++4e-=2H2 阳极:2H2O-4e-=4H++O2 LiNi x Co y Mn z O2=δLi++Li1-δ Ni x Co y Mn z O2+δe- 4OH--4e-=O2+2H2O | Li,Co,Ni,Mn:>98.0 | 25[ |
| 1 | WANG Yuqing, AN Ning, WEN Lei, et al. Recent progress on the recycling technology of Li-ion batteries[J]. Journal of Energy Chemistry, 2021, 55: 391-419. |
| 2 |
CHANG Xin, FAN Min, GU Chaofan, et al. Selective extraction of transition metals from spent LiNi x Co y Mn1- x- y O2 cathode via regulation of coordination environment[J]. Angewandte Chemie, 2022, 134(24).Doi:10.1002/ange.202202558 .
doi: 10.1002/ange.202202558 |
| 3 |
ZHAO Yunze, LIU Bingguo, ZHANG Libo, et al. Microwave pyrolysis of macadamia shells for efficiently recycling lithium from spent lithium-ion batteries[J]. Journal of Hazardous Materials, 2020, 396.Doi:10.1016/j.jhazmat.2020.122740 .
doi: 10.1016/j.jhazmat.2020.122740 |
| 4 |
XIAO Hougui, JI Guanjun, YE Long, et al. Efficient regeneration and reutilization of degraded graphite as advanced anode for lithi-um-ion batteries[J]. Journal of Alloys and Compounds, 2021, 888.Doi:10.1016/j.jallcom.2021.161593 .
doi: 10.1016/j.jallcom.2021.161593 |
| 5 |
LI Jun, LAI Yiming, ZHU Xianqing, et al. Pyrolysis kinetics and reaction mechanism of the electrode materials during the spent LiCoO2 batteries recovery process[J]. Journal of Hazardous Materials, 2020, 398.Doi:10.1016/j.jhazmat.2020.122955 .
doi: 10.1016/j.jhazmat.2020.122955 |
| 6 | FAN Ersha, LI Li, WANG Zhenpo, et al. Sustainable recycling technology for Li-ion batteries and beyond:Challenges and future prospects[J]. Chemical Reviews, 2020, 120(14):7020-7063. |
| 7 |
CHEN Dongdong, RAO Shuai, WANG Dongxing, et al. Synergistic leaching of valuable metals from spent Li-ion batteries using sulfuric acid-l-ascorbic acid system[J]. Chemical Engineering Journal, 2020, 388.Doi:10.1016/j.cej.2020.124321 .
doi: 10.1016/j.cej.2020.124321 |
| 8 | 钟雪虎, 陈玲玲, 韩俊伟, 等. 废旧锂离子电池资源现状及回收利用[J]. 工程科学学报, 2021, 43(2):161-169. |
| ZHONG Xuehu, CHEN Lingling, HAN Junwei, et al. Overview of present situation and technologies for the recovery of spent lithium-ion batteries[J]. Chinese Journal of Engineering, 2021, 43(2):161-169. | |
| 9 |
XIAO Jiefeng, NIU Bo, SONG Qingming, et al. Novel targetedly extracting lithium:An environmental-friendly controlled chlorinating technology and mechanism of spent lithium ion batteries reco-very[J]. Journal of Hazardous Materials, 2021, 404.Doi:10.1016/j.jhazmat.2020123947 .
doi: 10.1016/j.jhazmat.2020123947 |
| 10 |
XIAO Jiefeng, GAO Ruitong, NIU Bo, et al. Study of reaction characteristics and controlling mechanism of chlorinating conversion of cathode materials from spent lithium-ion batteries[J]. Journal of Hazardous Materials, 2021, 407.Doi:10.1016/j.jhazmat.2020.124704 .
doi: 10.1016/j.jhazmat.2020.124704 |
| 11 |
XIAO Jiefeng, NIU Bo, XU Zhenming. Highly efficient selective recovery of lithium from spent lithium-ion batteries by thermal reduction with cheap ammonia reagent[J]. Journal of Hazardous Materials, 2021, 418.Doi:10.1016/j.jhazmat.2021.126319 .
doi: 10.1016/j.jhazmat.2021.126319 |
| 12 |
HU Xianfeng, MOUSA E, TIAN Yang, et al. Recovery of Co,Ni,Mn,and Li from Li-ion batteries by smelting reduction-Part I:A laboratory-scale study[J]. Journal of Power Sources, 2021, 483.Doi:10.1016/j.jpowsour.2020.228936 .
doi: 10.1016/j.jpowsour.2020.228936 |
| 13 |
HU Xianfeng, MOUSA E, YE Guozhu. Recovery of Co,Ni,Mn,and Li from Li-ion batteries by smelting reduction-Part Ⅱ:A pilot-scale demonstration[J]. Journal of Power Sources, 2021, 483.Doi:10.1016/j.jpowsour.2020.229089 .
doi: 10.1016/j.jpowsour.2020.229089 |
| 14 |
TANG Yiqi, QU Xin, ZHANG Beilei, et al. Recycling of spent lithium nickel cobalt manganese oxides via a low-temperature ammonium sulfation roasting approach[J]. Journal of Cleaner Production, 2021, 279.Doi:10.1016/j.jclepro.2020.123633 .
doi: 10.1016/j.jclepro.2020.123633 |
| 15 | HOREH N B, MOUSAVI S M, SHOJAOSADATI S A. Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus Niger[J]. Journal of Power Sources, 2016, 320: 257-266. |
| 16 | JEGAN ROY J, SRINIVASAN M, CAO Bin. Bioleaching as an eco-friendly approach for metal recovery from spent NMC-based lithium-ion batteries at a high pulp density[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(8):3060-3069. |
| 17 |
XING Lei, BAO Jieru, ZHOU Shiyu, et al. Ultra-fast leaching of critical metals from spent lithium-ion batteries cathode materials achieved by the synergy-coordination mechanism[J]. Chemi-cal Engineering Journal, 2021, 420.Doi:10.1016/j.cej.2021.129593 .
doi: 10.1016/j.cej.2021.129593 |
| 18 | LI Li, ZHAI Longyu, ZHANG Xiaoxiao, et al. Recovery of valuable metals from spent lithium-ion batteries by ultrasonic-assisted leaching process[J]. Journal of Power Sources, 2014, 262: 380-385. |
| 19 |
GU Shuai, KONG Jiao, XING Lei, et al. Insights into the coordination enhanced leaching mechanism of spent lithium-ion batteries cathode materials[J]. Journal of Environmental Chemical Engineering, 2022, 10(3).Doi:10.1016/j.jece.2022.107745 .
doi: 10.1016/j.jece.2022.107745 |
| 20 | LI Shuzhen, WU Xin, JIANG Youzhou, et al. Novel electrochemically driven and internal circulation process for valuable metals recycling from spent lithium-ion batteries[J]. Waste Management, 2021, 136: 18-27. |
| 21 |
JANG Y, HOU C H, PARK S, et al. Direct electrochemical lithium recovery from acidic lithium-ion battery leachate using intercalation electrodes[J]. Resources,Conservation and Recycling, 2021, 175.Doi:10.1016/j.resconrec.2021.105837 .
doi: 10.1016/j.resconrec.2021.105837 |
| 22 | 赵鹏飞, 尹晓莹, 满瑞林. 电解法在废旧锂电池浸出过程中的应用[J]. 中国有色冶金, 2014, 43(5):73-78. |
| ZHAO Pengfei, YIN Xiaoying, MAN Ruilin. Application of electrolytic method in spent Li-ion battery leaching process[J]. China Nonferrous Metallurgy, 2014, 43(5):73-78. | |
| 23 | MENG Qi, ZHANG Yingjie, DONG Peng. Use of electrochemical cathode-reduction method for leaching of cobalt from spent lithi-um-ion batteries[J]. Journal of Cleaner Production, 2018, 180: 64-70. |
| 24 |
ZHOU Siyuan, ZHANG Yingjie, MENG Qi, et al. Recycling of spent LiCoO2 materials by electrolytic leaching of cathode electrode plate[J]. Journal of Environmental Chemical Engineering, 2021, 9(1).Doi:10.1016/j.jece.2020.104789 .
doi: 10.1016/j.jece.2020.104789 |
| 25 |
DANG Sen, HOU Wei, MIN Yulin, et al. Electro-oxidation:A win-win strategy for the selective recovery of Li+ from spent lithium-ion batteries and the preparation of highly active catalysts[J]. Chemical Engineering Journal, 2022, 435.Doi:10.1016/j.cej.2022.135169 .
doi: 10.1016/j.cej.2022.135169 |
| 26 |
LV Hong, HUANG Haijian, HUANG Cheng, et al. Electric field driven de-lithiation:A strategy towards comprehensive and efficient recycling of electrode materials from spent lithium ion batteries[J]. Applied Catalysis B:Environmental, 2021, 283.Doi:10.1016/j.apcatb.2020.119634 .
doi: 10.1016/j.apcatb.2020.119634 |
| 27 | LIU Kui, YANG Shenglong, LAI Feiyan, et al. Innovative electrochemical strategy to recovery of cathode and efficient lithium leaching from spent lithium-ion batteries[J]. ACS Applied Energy Materials, 2020, 3(5):4767-4776. |
| 28 | ZHANG Beilei, QU Xin, QU Jiakang, et al. A paired electrolysis approach for recycling spent lithium iron phosphate batteries in an undivided molten salt cell[J]. Green Chemistry, 2020, 22(24):8633-8641. |
| 29 | HUA Yunhui, XU Zhenghe, ZHAO Baojun, et al. Electric potential-determined redox intermediates for effective recycling of spent lithium-ion batteries[J]. Green Chemistry, 2022, 24(9):3723-3735. |
| 30 |
LV Weiguang, RUAN Dingshan, ZHENG Xiaohong, et al. One-step recovery of valuable metals from spent lithium-ion batteries and synthesis of persulfate through paired electrolysis[J]. Chemical Engineering Journal, 2021, 421.Doi:10.1016/j.cej.2021.129908 .
doi: 10.1016/j.cej.2021.129908 |
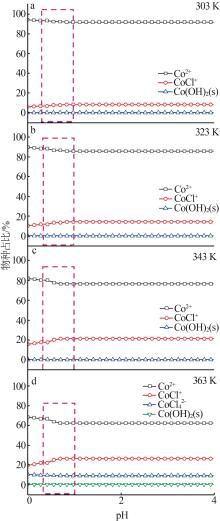
| 31 | MIGDISOV A A, ZEZIN D, WILLIAMS-JONES A E. An experimental study of Cobalt(Ⅱ) complexation in Cl- and H2S-bearing hydrothermal solutions[J]. Geochimica et Cosmochimica Acta, 2011, 75(14):4065-4079. |
| 32 | PAN Pujing, SUSAK N J. Co(Ⅱ)-chloride and-bromide complexes in aqueous solutions up to 5 m NaX and 90 ℃:Spectrophotometric study and geological implications[J]. Geochimica et Cosmochimica Acta, 1989, 53(2):327-341. |
| 33 | LIU Weihua, BORG S J, TESTEMALE D, et al. Speciation and thermodynamic properties for cobalt chloride complexes in hydrothermal fluids at 35~440 ℃ and 600 bar:An in situ XAS study[J]. Geochimica et Cosmochimica Acta, 2011, 75(5):1227-1248. |
| 34 | HUANG T C, TSAI T H. Extraction equilibrium of cobalt(Ⅱ) from sulphate solutions by di(2-etrylhexyl)phosphoric acid dissolved in kerosene[J]. Polyhedron, 1990, 9(9):1147-1153. |
| 35 |
GU Shuai, ZHANG Liang, FU Bitian, et al. Recycling of mixed lithium-ion battery cathode materials with spent lead-acid battery electrolyte with the assistance of thermodynamic simulations[J]. Journal of Cleaner Production, 2020, 266.Doi:10.1016/j.jclepro.2020.121827 .
doi: 10.1016/j.jclepro.2020.121827 |
| 36 | WANG Mengmeng, ZHANG Congcong, ZHANG Fushen. An environmental benign process for cobalt and lithium recovery from spent lithium-ion batteries by mechanochemical approach[J]. Waste Management, 2016, 51: 239-244. |
| 37 | 赵中伟, 霍广生. Li-Mn-H2O系热力学分析[J]. 中国有色金属学报, 2004, 14(11):1926-1933. |
| ZHAO Zhongwei, HUO Guangsheng. Thermodynamic analysis of Li-Mn-H2O system[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(11):1926-1933. | |
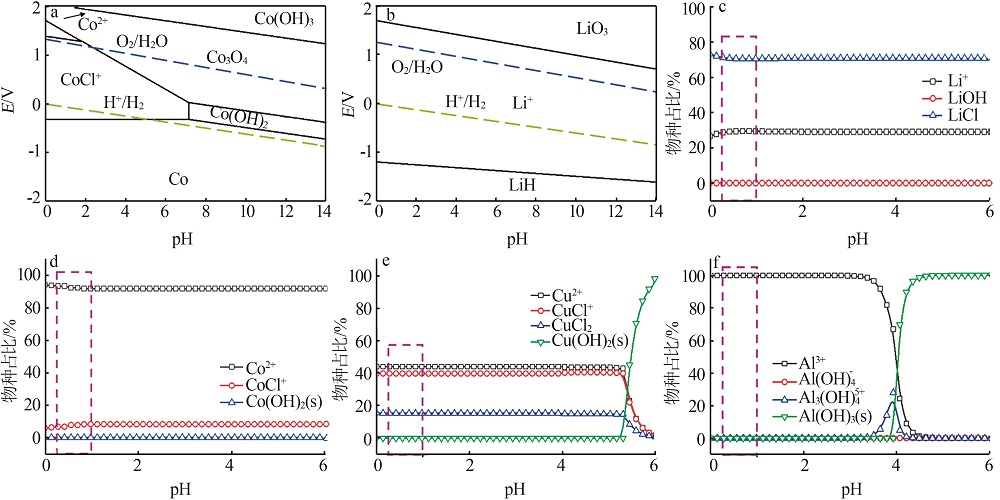
| 38 | 文士美, 赵中伟, 霍广生. Li-Co-H2O系热力学分析及E-pH图[J]. 电源技术, 2005, 29(7):423-426. |
| WEN Shimei, ZHAO Zhongwei, HUO Guangsheng. Thermodynamic analysis and potential-pH diagrams of Li-Co-H2O system[J]. Chinese Journal of Power Sources, 2005, 29(7):423-426. | |
| 39 | 郭持皓, 赵中伟, 霍广生. Li-Ni-H2O系的热力学分析[J]. 电源技术, 2005, 29(6):376-379. |
| GUO Chihao, ZHAO Zhongwei, HUO Guangsheng. Thermodyna-mic analysis on Li-Ni-H2O system[J]. Chinese Journal of Power Sources, 2005, 29(6):376-379. | |
| 40 | WANG Hongmin, LI Mengran, GARG S, et al. Cobalt electroche-mical recovery from lithium cobalt oxides in deep eutectic choline Chloride+Urea solvents[J]. ChemSusChem, 2021, 14(14):2972-2983. |
| 41 | PRABAHARAN G, BARIK S P, KUMAR N, et al. Electrochemical process for electrode material of spent lithium ion batteries[J]. Waste Management, 2017, 68: 527-533. |
| 42 | HE Lipo, SUN Shuying, SONG Xingfu, et al. Leaching process for recovering valuable metals from the LiNi1/3Co1/3Mn1/3O2 cathode of lithium-ion batteries[J]. Waste Management, 2017, 64: 171- 181. |
| 43 | HE Yaqun, ZHANG Tao, WANG Fangfang, et al. Recovery of LiCoO2 and graphite from spent lithium-ion batteries by Fenton reagent-assisted flotation[J]. Journal of Cleaner Production, 2017, 143: 319-325. |
| 44 | MAY M, KUO J, TAN C T. Vacuum electrolysis reactor technique for quantitation of 13-carbon isotope enrichment at the C1-position of formic acid and acetic acid[J]. Analytical Chemistry, 2004, 76(18):5313-5318. |
| 45 | NAIR S M K, JAMES C. Numerical data for the commonly used solid-state reaction equations[J]. Thermochimica Acta, 1985, 83(2):387-389. |
| 46 | DICKINSON C F, HEAL G R. Solid-liquid diffusion controlled rate equations[J]. Thermochimica Acta, 1999, 340-341: 89- 103. |
| 47 | LI Li, FAN Ersha, GUAN Yibiao, et al. Sustainable recovery of cathode materials from spent lithium-ion batteries using lactic acid leaching system[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(6):5224-5233. |
| 48 | FEAR C, JUAREZ-ROBLES D, JEEVARAJAN J A, et al. Elucidating copper dissolution phenomenon in Li-ion cells under overdischarge extremes[J]. Journal of the Electrochemical Society, 2018, 165(9):A1639-A1647. |
| 49 | CIFUENTES G, HERNÁNDEZ J, GUAJARDO N. Recovering scrap anode copper using reactive electrodialysis[J]. American Journal of Analytical Chemistry, 2014, 5(15):1020-1027. |
| 50 | KOGA T, NONAKA K, SAKATA Y, et al. Electrochemical formation and accumulation of Cu(Ⅰ) in copper sulfate electroplating solution[J]. Journal of the Electrochemical Society, 2018, 165(10):D423-D426. |
| [1] | TIAN Xiaoli, LI Zhixun, FENG Runtang, ZHANG Jie, ZHENG Quanfu, SHI Xuwu, DU Yongbin. Study on thermal decomposition behavior of Tibetan Kamado microcrystalline magnesite [J]. Inorganic Chemicals Industry, 2023, 55(3): 60-65. |
| [2] | ZHOU Shiyu,HE Ting,FU Tongtong,GUO Zirui,GU Shuai,YU Jianguo. Life cycle and economic assessment of recycling spent lithium-ion batteries with hydrometallurgical process [J]. Inorganic Chemicals Industry, 2023, 55(1): 26-32. |
| [3] | Liu Zhiguo,Zhang Na,Chen Chuan,Xu Xianzhen. Study on thermodynamic model of vapor-liquid equilibrium of electrolyte system [J]. Inorganic Chemicals Industry, 2021, 53(8): 55-59. |
| [4] | Liang Ning,Mo Fujin,Zhou Jierong,Wang Junzheng. Study on preparation of sludge biochar and its adsorption performance for phosphorus [J]. Inorganic Chemicals Industry, 2021, 53(6): 174-179. |
| [5] | Li Fei,Zhou Jianmin,Li Chengyu,Xu Wentao,Zhang Xinyue,Li Xinwei,Wang Jun,Ji Zhiyong,Zhao Yingying,Guo Xiaofu,Yuan Junsheng. Study on mechanism and conditions of the new process of ammonium chloride conversion [J]. Inorganic Chemicals Industry, 2021, 53(3): 48-53. |
| [6] | LIANG Chengbo,XIE Tian,YANG Lin,HE Bingbing. Study on leaching law of metal elements in process of decomposing phosphate rock by phosphoric acid [J]. Inorganic Chemicals Industry, 2021, 53(11): 49-54. |
| [7] | Liu Juexin,Zheng Chenggang,Ye Shichao. Determination and application of thermodynamic data of potassium dihydrogen phosphate crystal [J]. Inorganic Chemicals Industry, 2021, 53(1): 62-64. |
| [8] | Wang Haohao,Da Tao,Ye Shichao. Experimental study on crystallization process of ammonium chloride [J]. Inorganic Chemicals Industry, 2020, 52(4): 49-52. |
| [9] | Li Ying,Chen Xia,Miao Shulan,Liao Enxin. Study on adsorption properties of ammonium phosphate tungsten composites on rubidium and cesium [J]. Inorganic Chemicals Industry, 2019, 51(6): 34-37. |
| [10] | . Thermodynamic analysis on system of ZnO-NH3-NH4HCO3-H2O in process of zinc oxide leaching [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(6): 30-. |
| [11] | SUN Chong, CHEN Xue-An, CHANG Xin-An, XIAO Wei-Qiang, WANG Shao-Hua, CHEN Yan-Jun. Study on acid leaching conditions of Ni from tailings [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(8): 49-. |
| [12] | KANG Qi-Yu, DING Wei-Jie, XIAO Wen-De, YAN Jian-Min, LUO Man. Thermodynamic analysis of co-hydrogenation of silicon tetrachloride and silicon [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(11): 26-. |
| [13] | Meng Jun;Liu Gousheng;Wang Lijuan;Zhao Hongyu;Yu Jianguo. Thermodynamics analysis on carbon reducing reaction of strontium sulfate [J]. INORGANICCHEMICALSINDUSTRY, 2009, 0(3): 0-0. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||