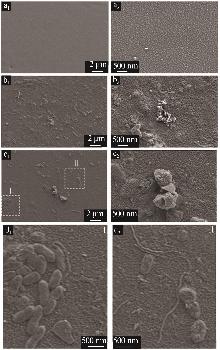
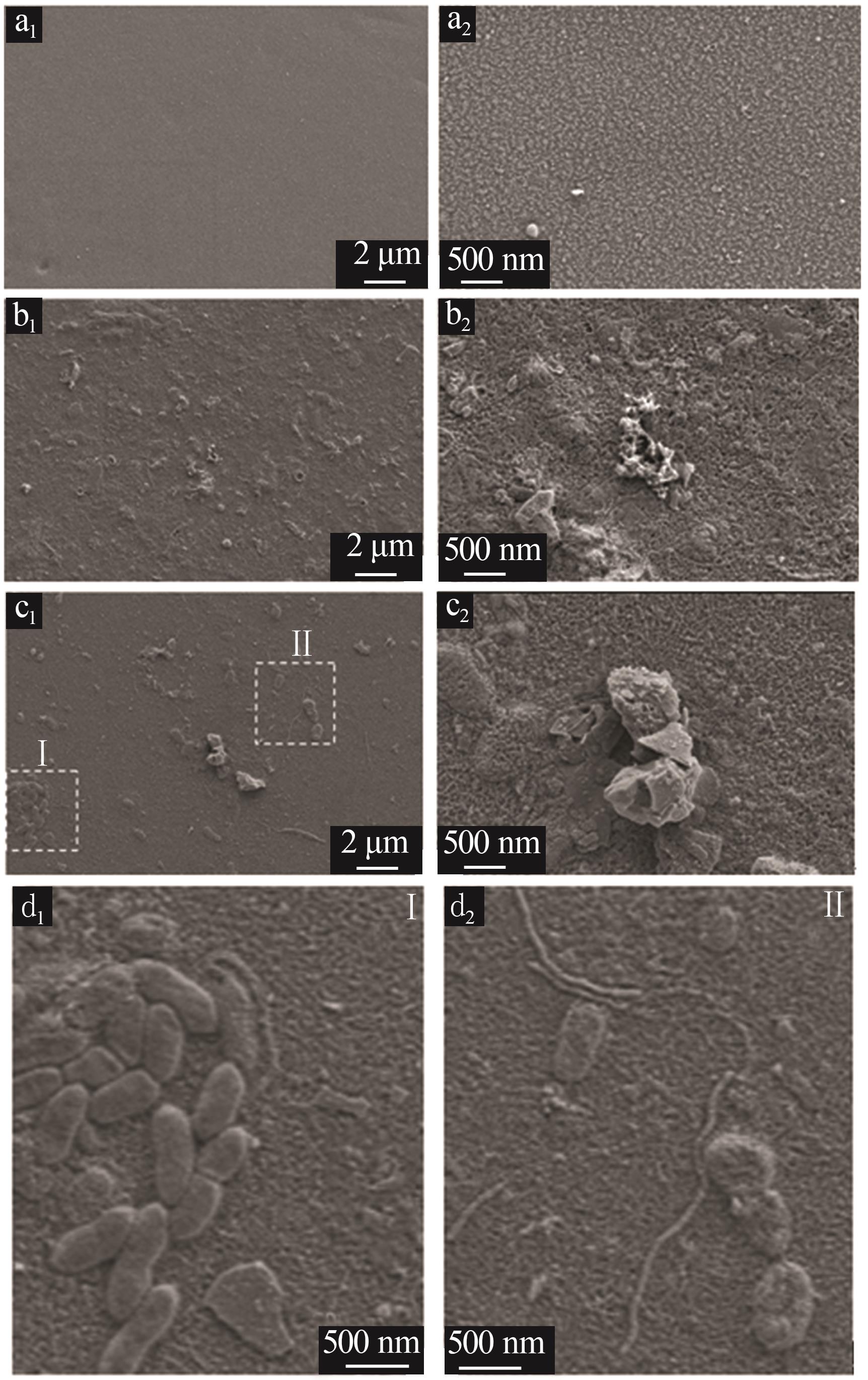
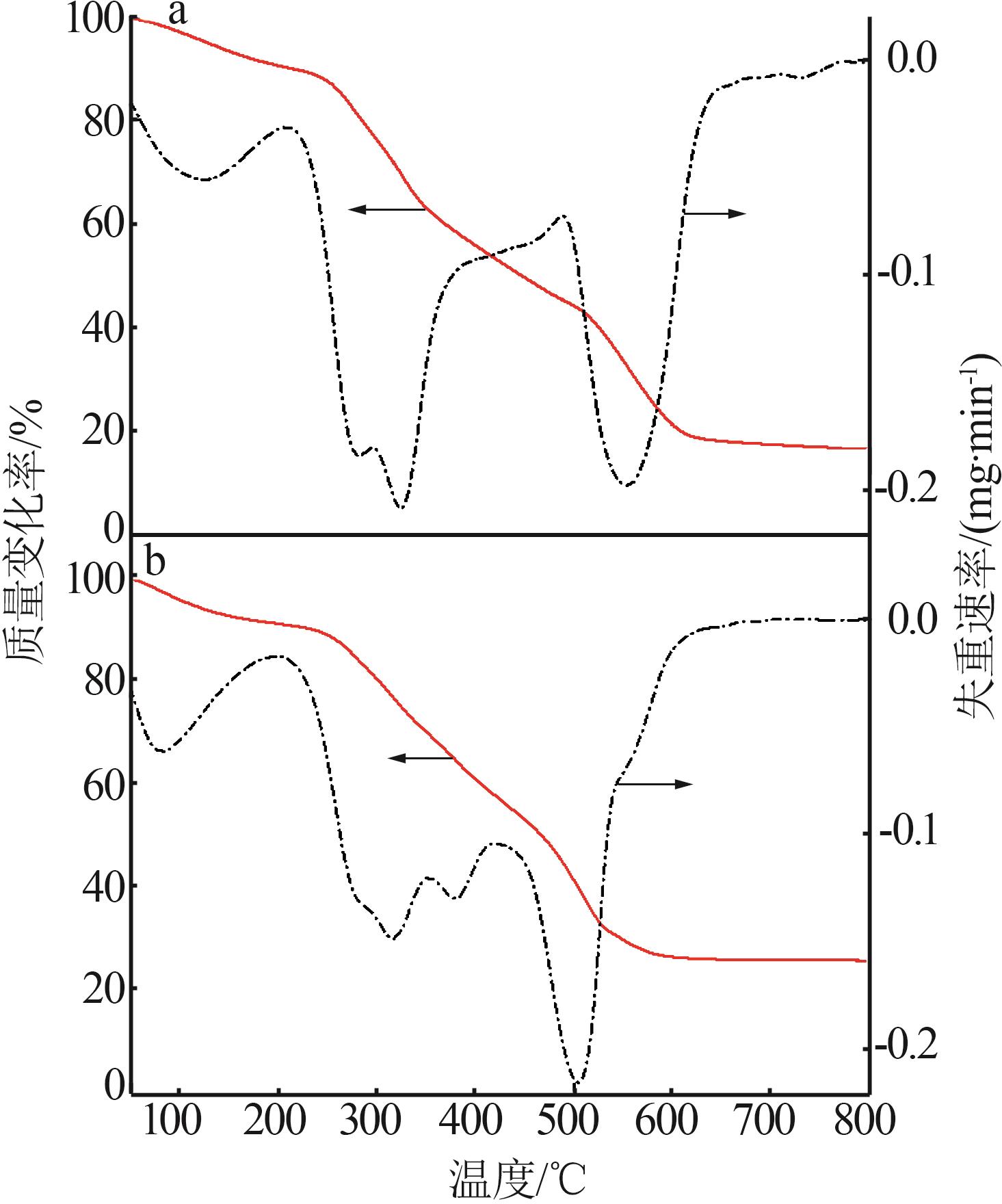
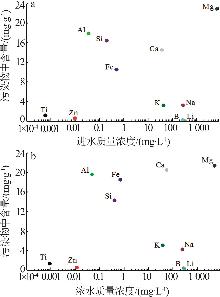
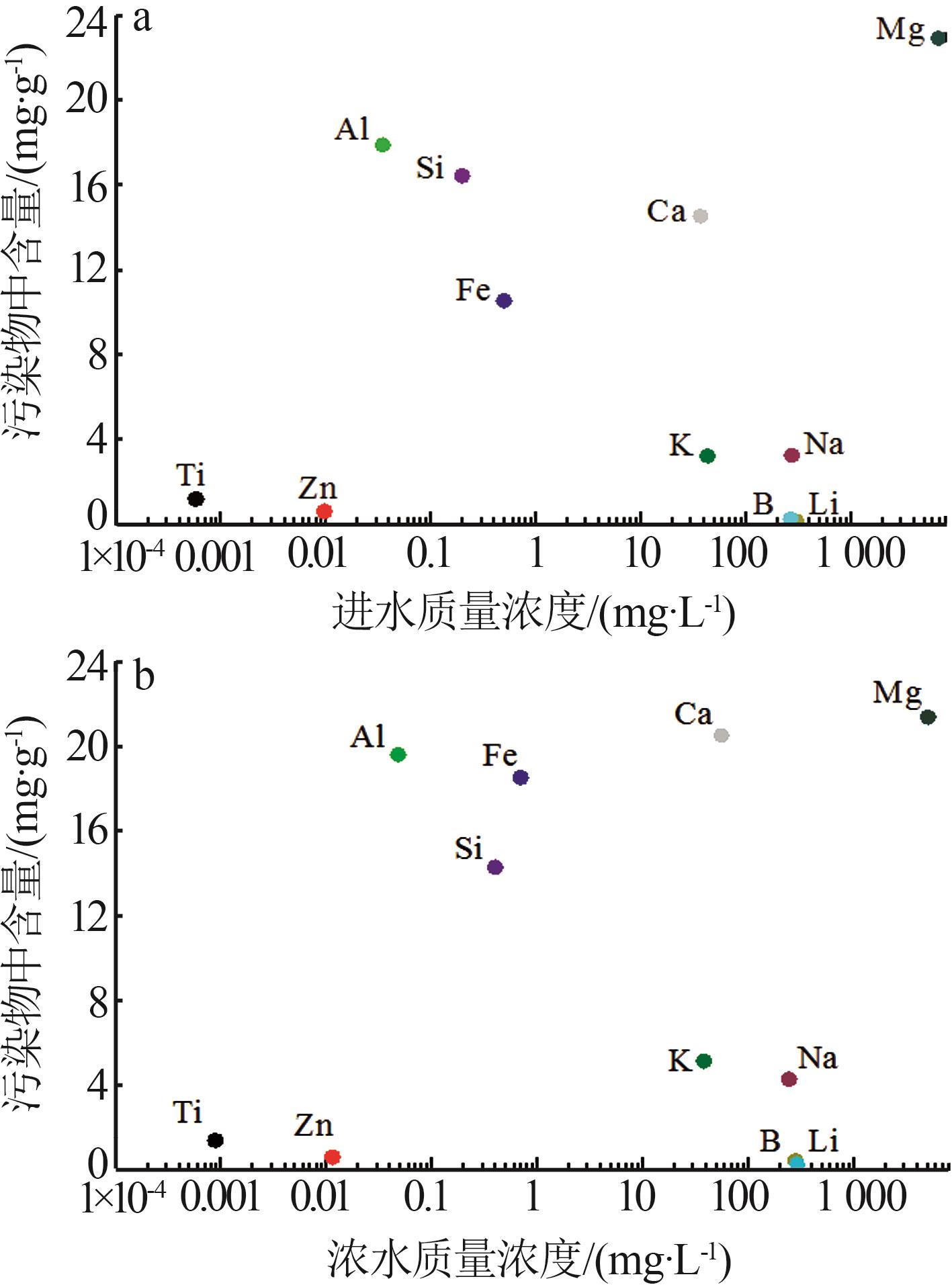
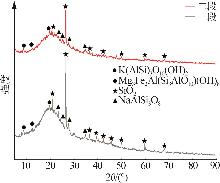
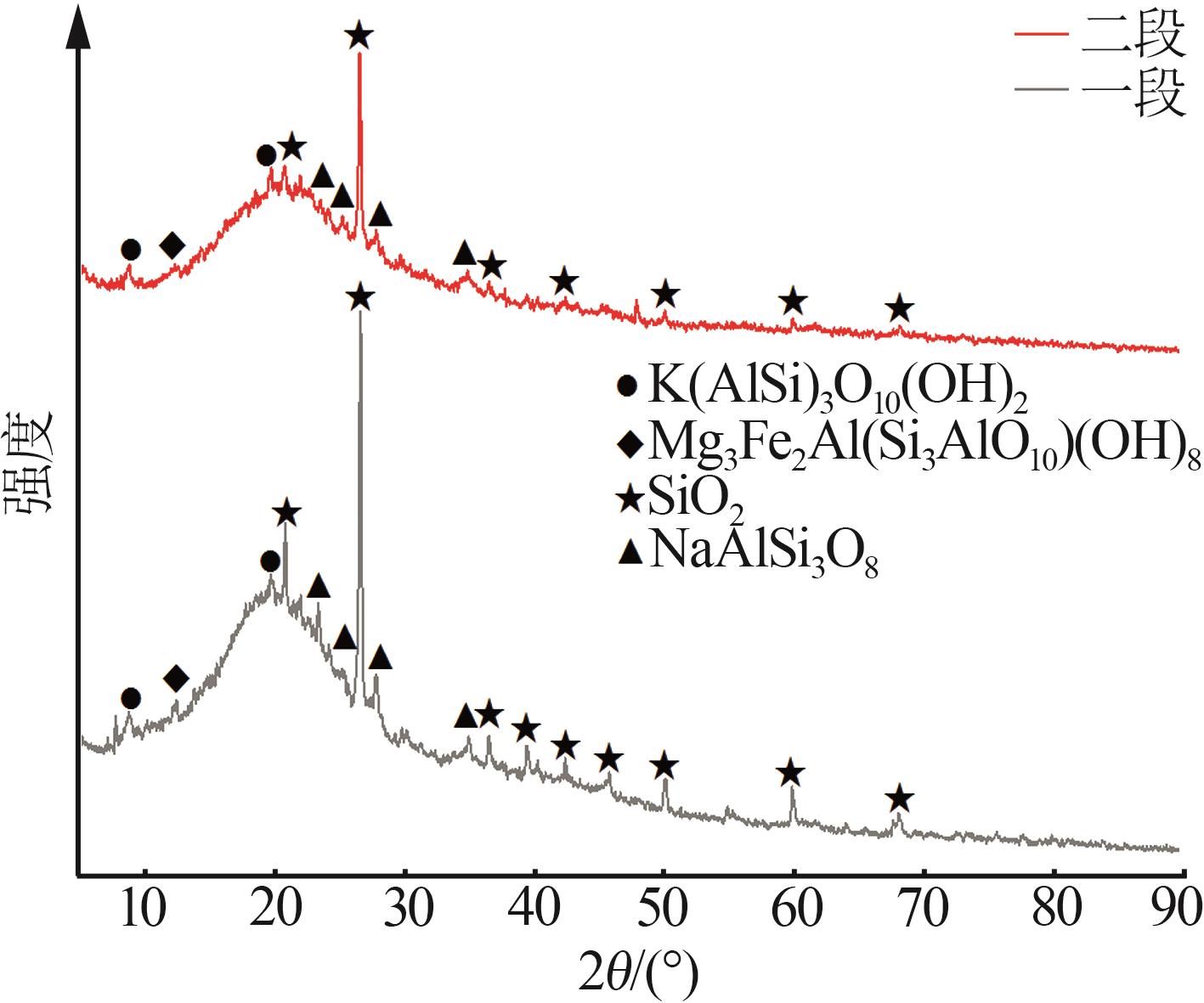
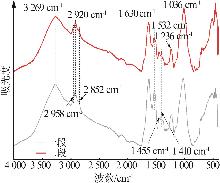
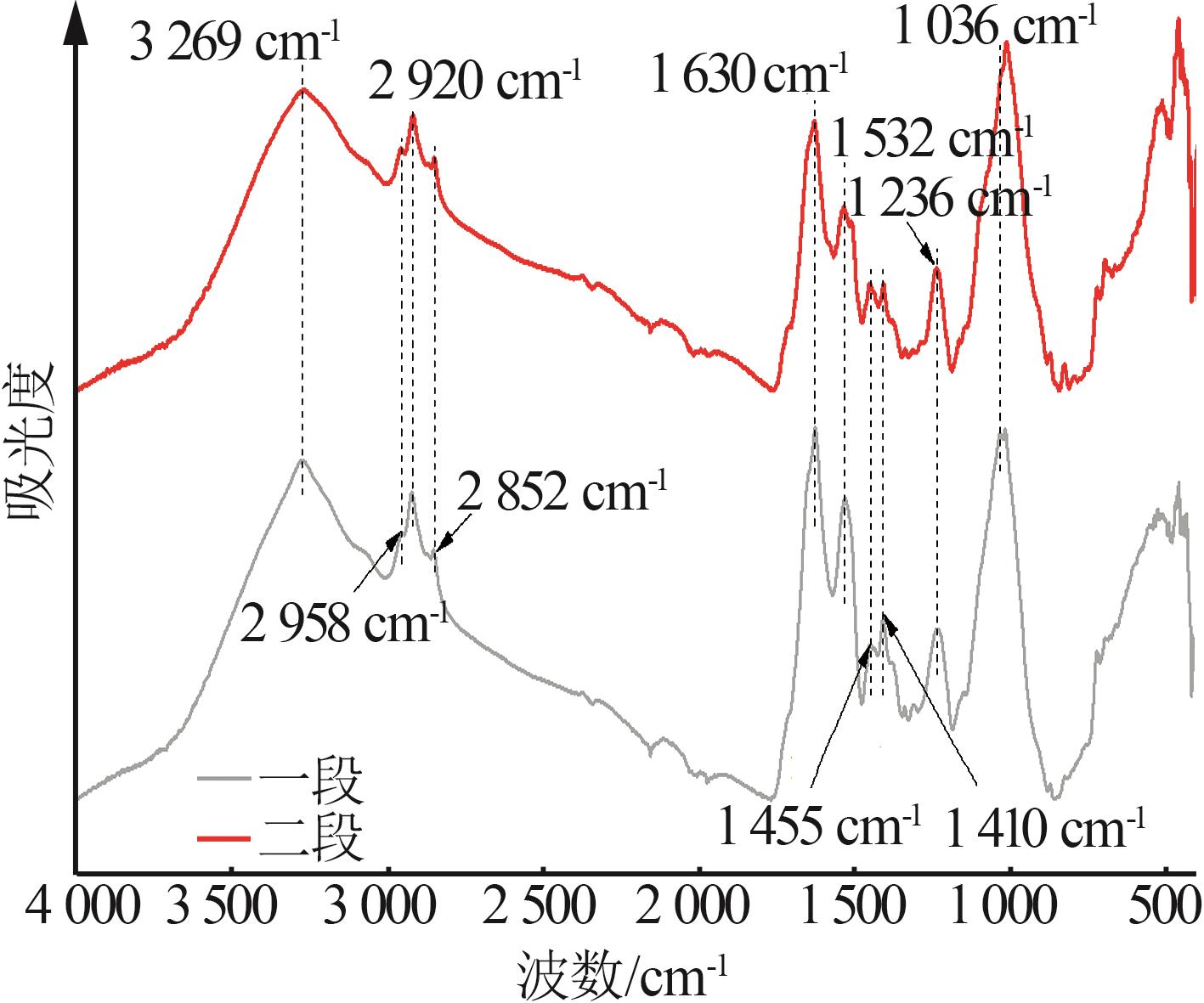
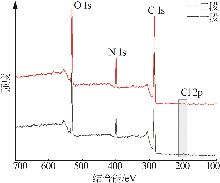
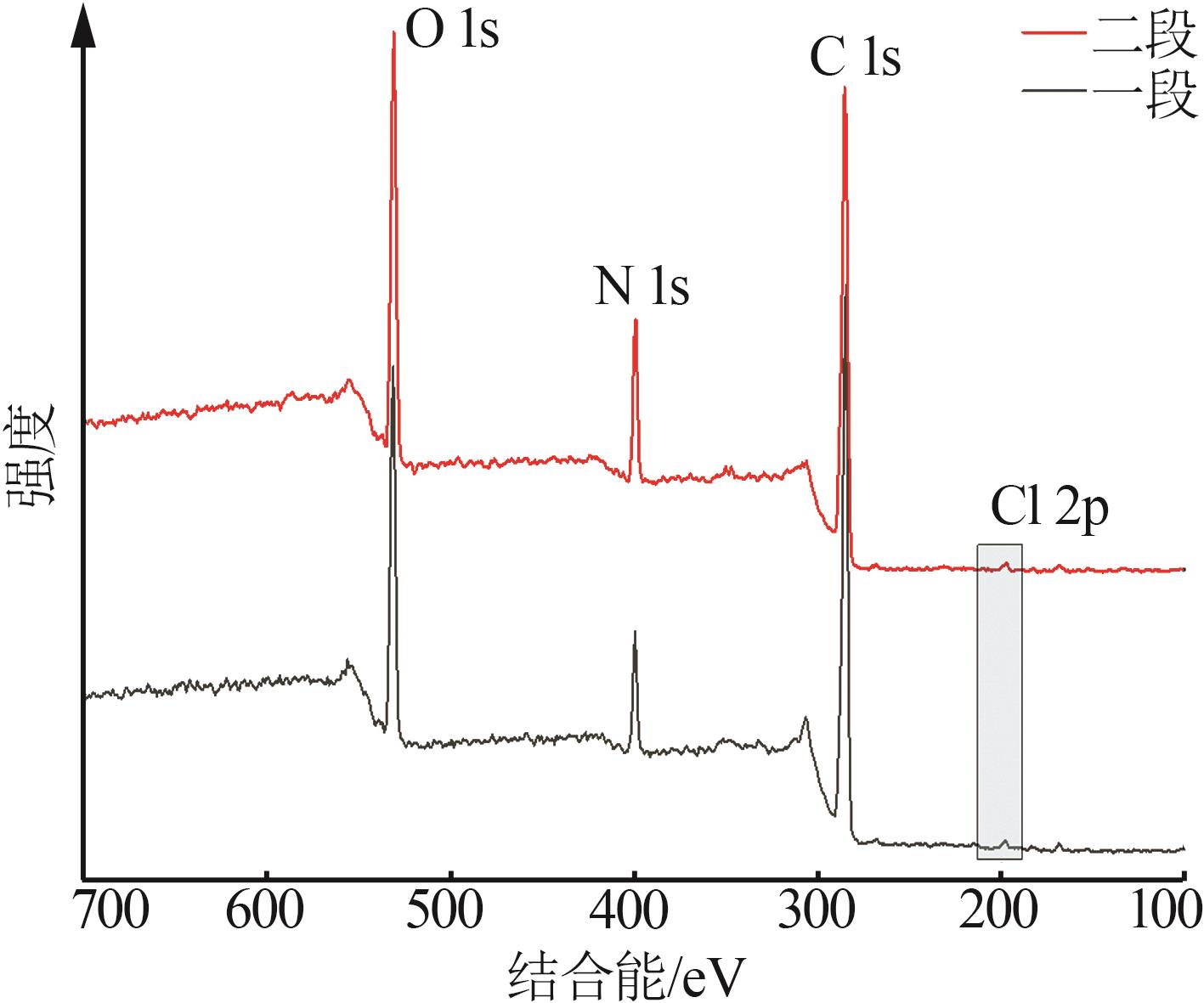
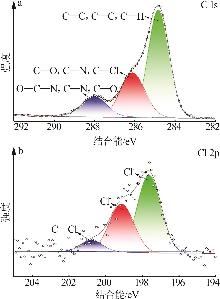
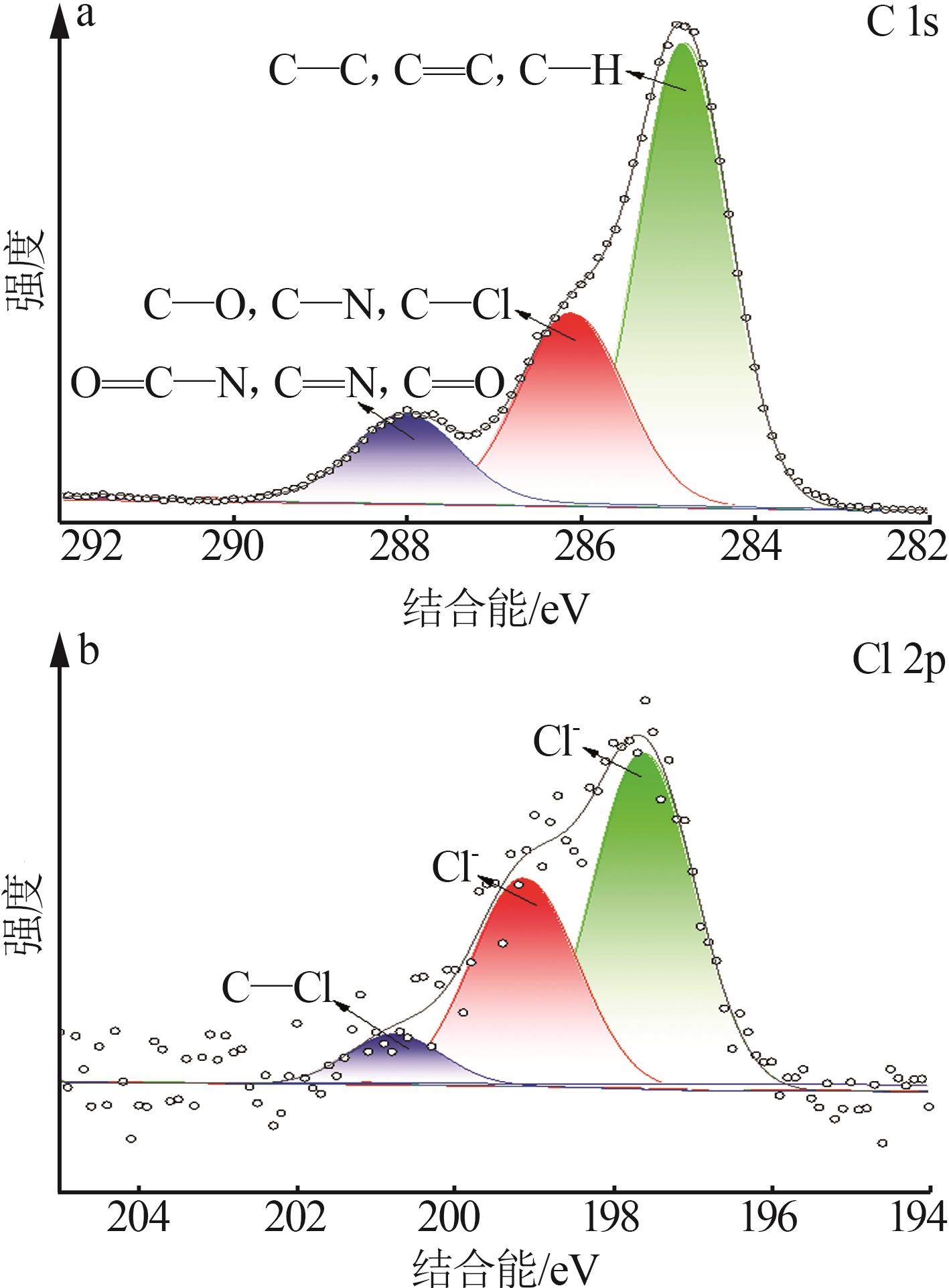
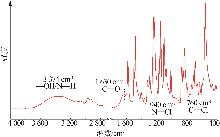
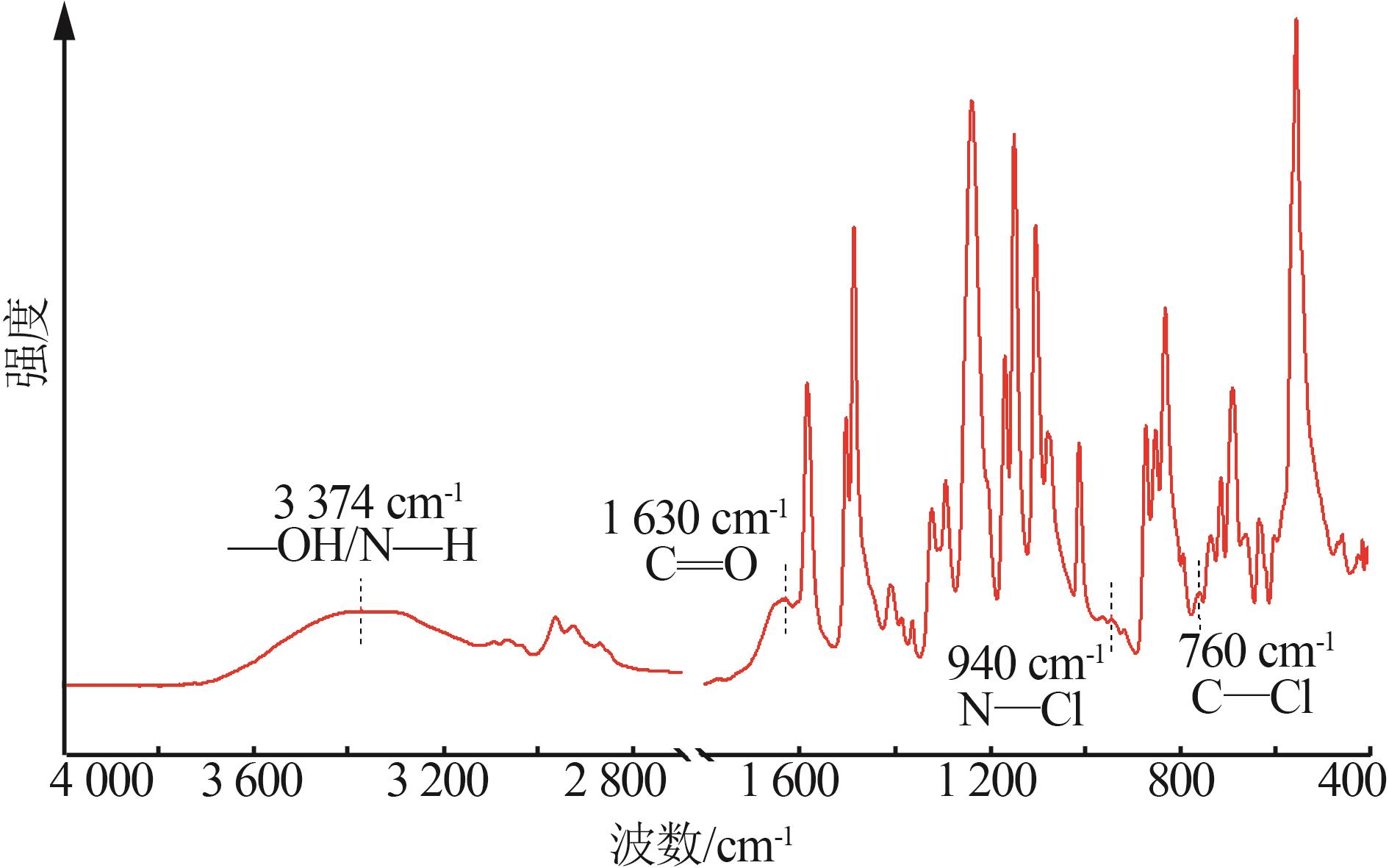
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (10): 90-97.doi: 10.19964/j.issn.1006-4990.2024-0402
• Industrial Techniques • Previous Articles Next Articles
Degradation analysis of nanofiltration membrane performance for Qinghai Salt Lake
ZHANG Shengpeng1( ), HUANG Minmin2(
), HUANG Minmin2( ), TANG Faman1, LIU Weiping1
), TANG Faman1, LIU Weiping1
- 1. Minmetals Salt Lake Co. ,Ltd. ,Xining 810000,China
2. Hangzhou Water Treatment Technology Development Center,Hangzhou 310012,China
-
Received:2024-07-11Online:2025-10-10Published:2024-09-11 -
Contact:HUANG Minmin E-mail:zhangsp21@163.com;huangminmin@chinawatertech.com
CLC Number:
Cite this article
ZHANG Shengpeng, HUANG Minmin, TANG Faman, LIU Weiping. Degradation analysis of nanofiltration membrane performance for Qinghai Salt Lake[J]. Inorganic Chemicals Industry, 2025, 57(10): 90-97.
share this article
| [1] | 孔令杰,李光壁,谢佳豪,等.盐湖卤水锂提取技术研究进展[J].无机盐工业,2025,57(1):14-26. |
| KONG Lingjie, LI Guangbi, XIE Jiahao,et al.Research progress on lithium extraction technology from salt lake brine[J].Inorganic Chemicals Industry,2025,57(1):14-26. | |
| [2] | 林钰青,张以任,邱宇隆,等.膜技术在盐湖提锂中的进展和展望[J].无机盐工业,2023,55(1):33-45. |
| LIN Yuqing, ZHANG Yiren, QIU Yulong,et al.Progress and prospect of membrane technology in lithium extraction from salt lake brine[J].Inorganic Chemicals Industry,2023,55(1):33-45. | |
| [3] | 许家晟,谢建国,鹿燕,等.碟管式纳滤膜在生活用水处理中的膜污染特性[J].化工进展,2020,39(5):2000-2008. |
| XU Jiasheng, XIE Jianguo, LU Yan,et al.Membrane fouling characteristics of disk-tube nanofiltration membrane to domestic water treatment[J].Chemical Industry and Engineering Progress,2020,39(5):2000-2008. | |
| [4] | 刘丹阳,赵尔卓,仲丽娟,等.低压纳滤膜用于微污染地表水深度处理的中试研究[J].给水排水,2019,55(4):15-23. |
| LIU Danyang, ZHAO Erzhuo, ZHONG Lijuan,et al.Pilot study for the treatment of micro-polluted surface water by using a low-pressure nanofiltration membrane system[J].Water & Wastewater Engineering,2019,55(4):15-23. | |
| [5] | FAN Chengyue, YAN Jiaming, LIU Haodong,et al.Performance and membrane fouling characteristics of a drinking water multistage NF system based on membrane autopsy from a full-scale system[J].Journal of Water Process Engineering,2024,58:104909. |
| [6] | GORZALSKI A S, CORONELL O.Fouling of nanofiltration membranes in full- and bench-scale systems treating groundwater containing silica[J].Journal of Membrane Science,2014,468:349- 359. |
| [7] | BEYER F, RIETMAN B M, ZWIJNENBURG A,et al.Long-term performance and fouling analysis of full-scale direct nanofiltration (NF) installations treating anoxic groundwater[J].Journal of Membrane Science,2014,468:339-348. |
| [8] | XU Rui, QIN Wei, ZHANG Bing,et al.Nanofiltration in pilot scale for wastewater reclamation:Long-term performance and membrane biofouling characteristics[J].Chemical Engineering Journal,2020, 395:125087. |
| [9] | 王金燕,王曼曼,李鸽,等.盐湖提锂用退役纳滤膜的污染分析[J].膜科学与技术,2023,43(5):83-88. |
| WANG Jinyan, WANG Manman, LI Ge,et al.Fouling characteristics of retired nanofiltration membranes for lithium extraction from salt-lake brines[J].Membrane Science and Technology,2023,43(5):83-88. | |
| [10] | 黄河清.聚哌嗪酰胺复合纳滤膜的氯化特性和耐氯改性研究[D].西安:西安建筑科技大学,2023. |
| HUANG Heqing.Study on chlorination characteristics and chlorine-resistant modification of polypiperazine amide compostie nanofiltration membrane[D].Xi′an:Xi′an University of Architecture and Technology,2023. | |
| [11] | GAO Lin, WANG Huaiyou, ZHANG Yue,et al.Nanofiltration membrane characterization and application:Extracting lithium in lepidolite leaching solution[J].Membranes,2020,10(8):178. |
| [12] | 周晓东,吴浩,刘景梅,等.TG-FTIR研究煤油共热解产物逸出行为[J].燃料化学学报(中英文),2024,52(4):525-537. |
| ZHOU Xiaodong, WU Hao, LIU Jingmei,et al.TG-FTIR study on escape behavior of products from co-pyrolysis of coal and residuum[J].Journal of Fuel Chemistry and Technology,2024,52(4):525-537. | |
| [13] | YU Tong, MENG Lu, ZHAO Qingbo,et al.Effects of chemical cleaning on RO membrane inorganic,organic and microbial foulant removal in a full-scale plant for municipal wastewater reclamation[J].Water Research,2017,113:1-10. |
| [14] | 李子浩.基于膜污染组分识别的再生水处理反渗透膜清洗技术优化研究[D].青岛:青岛理工大学,2023. |
| LI Zihao.Optimization of reverse osmosis membrane cleaning technology for wastewater reclamation treatment based on membrane fouling component identification[D].Qingdao:Qingdao University of Technology,2023. | |
| [15] | TANG Fang, HU Hongying, SUN Lijuan,et al.Fouling characteristics of reverse osmosis membranes at different positions of a full-scale plant for municipal wastewater reclamation[J].Water Research,2016,90:329-336. |
| [16] | KIM S J, OH B S, YU H W,et al.Foulant characterization and distribution in spiral wound reverse osmosis membranes from different pressure vessels[J].Desalination,2015,370:44-52. |
| [17] | TONG Xin, CUI Yong, WANG Yunhong,et al.Fouling properties of reverse osmosis membranes along the feed channel in an industrial-scale system for wastewater reclamation[J].Science of the Total Environment,2020,713:136673. |
| [18] | 丁成旺,马玉亮,陈建洲,等.柴达木盆地巴仑马海盐湖黏土沉积矿物学及稀有稀散元素地球化学特征[J].盐湖研究,2024,32(3):32-39. |
| DING Chengwang, MA Yuliang, CHEN Jianzhou,et al.Mineralogy and geochemical characteristics of rare elements in clays from Balun Mahai salt lake of Qaidam Basin[J].Journal of Salt Lake Research,2024,32(3):32-39. | |
| [19] | 张婷婷.膜技术深度处理维生素C生化出水的膜污染过程和特征研究[D].南京:南京大学,2014. |
| ZHANG Tingting.Study of the membrane fouling in advanced treating vitamin C secondary effluent by membrane technology[D].Nanjing:Nanjing University,2014. | |
| [20] | KHAN M T, BUSCH M, MOLINA V G,et al.How different is the composition of the fouling layer of wastewater reuse and seawater desalination RO membranes?[J].Water Research,2014,59:271-282. |
| [21] | 李梦晨.污水再生纳滤工艺中膜污染动态行为与过程解析[D].北京:清华大学,2018. |
| LI Mengchen.Dynamic behavior and evolution of nanofiltration membrane fouling during wastewater reclamation process[D].Beijing:Tsinghua University,2018. | |
| [22] | 翟姝.低浓度氧化剂与蛋白质的作用机理及其对膜污染控制效果的影响研究[D].南京:南京大学,2019. |
| ZHAI Shu.The study on reaction mechanisms of low dose oxidants with proteins and the effects on membrane fouling control[D].Nanjing:Nanjing University,2019. | |
| [23] | DREWS A.Membrane fouling in membrane bioreactors:Characterisation,contradictions,cause and cures[J].Journal of Membrane Science,2010,363(1/2):1-28. |
| [24] | 杨欢,高美荣,韩学凯,等.西藏五个盐湖浮游生物组成与微生物多样性[J].天津科技大学学报,2022,37(5):30-37. |
| YANG Huan, GAO Meirong, HAN Xuekai,et al.Planktonic composition and microbial biodiversity in five salt lakes in Tibet[J].Journal of Tianjin University of Science & Technology,2022,37(5):30-37. | |
| [25] | 计超,张杰,张志君,等.DK纳滤膜对高镁锂比卤水的分离性能研究[J].膜科学与技术,2014,34(3):79-85. |
| JI Chao, ZHANG Jie, ZHANG Zhijun,et al.Separation properties of magnesium and lithium from brine with high Mg2+/Li+ ratio by DK nanofiltration membrane[J].Membrane Science and Technology,2014,34(3):79-85. | |
| [26] | SUN Shuying, CAI Lijuan, NIE Xiaoyao,et al.Separation of magnesium and lithium from brine using a Desal nanofiltration membrane[J].Journal of Water Process Engineering,2015,7:210- 217. |
| [27] | LI Yan, ZHAO Youjing, WANG Huaiyou,et al.The application of nanofiltration membrane for recovering lithium from salt lake brine[J].Desalination,2019,468:114081. |
| [28] | 李梦菲,杨少霞,杨宏伟,等.哌嗪聚酰胺纳滤膜的氯氧化特性及机理[J].膜科学与技术,2023,43(1):27-36,55. |
| LI Mengfei, YANG Shaoxia, YANG Hongwei,et al.Chlorine oxidation characteristics and mechanism of piperazine polyamide nanofiltration membrane[J].Membrane Science and Technology,2023,43(1):27-36,55. | |
| [29] | TIN M M M, ANIOKE G, NAKAGOE O,et al.Membrane fouling,chemical cleaning and separation performance assessment of a chlorine-resistant nanofiltration membrane for water recycling applications[J].Separation and Purification Technology,2017,189:170-175. |
| [30] | DO V T, TANG C Y, REINHARD M,et al.Degradation of polyamide nanofiltration and reverse osmosis membranes by hypochlorite[J].Environmental Science & Technology,2012,46(2):852-859. |
| [31] | GHOLAMI S, REZVANI A, VATANPOUR V,et al.Improving the chlorine resistance property of polyamide TFC RO membrane by polyethylene glycol diacrylate(PEGDA) coating[J].Desalination,2018,443:245-255. |
| [32] | LI Y, WANG M, XIANG X,et al.Separation performance and fouling analyses of nanofiltration membrane for lithium extraction from salt lake brine[J].Journal of Water Process Engineering,2023,54:104009. |
| [33] | VERBEKE R, EYLEY S, SZYMCZYK A,et al.Controlled chlorination of polyamide reverse osmosis membranes at real scale for enhanced desalination performance[J].Journal of Membrane Science,2020,611:118400. |
| [34] | 魏新渝,王志.交联芳香聚酰胺复合反渗透膜氯化降解机理研究[C]//2011中国环境科学学会学术年会论文集(第四卷).乌鲁木齐,2011:875-880. |
| [35] | 闫露霞,孙美平,姚晓军,等.青藏高原湖泊水质变化及现状评价[J].环境科学学报,2018,38(3):900-910. |
| YAN Luxia, SUN Meiping, YAO Xiaojun,et al.Lake water in the Tibet Plateau:Quality change and current status evaluation[J].Acta Scientiae Circumstantiae,2018,38(3):900-910. |
| [1] | YANG Hengyu, MO Hengliang, LI Tianyu, LÜ Long, CHEN Yili, WANG Luocong, ZHAO Wenfang, LIU Manman. Study on synthesis of high acid resistant Ti-based lithium ion sieve using mixed crystal TiO2 as titanium source [J]. Inorganic Chemicals Industry, 2025, 57(7): 57-63. |
| [2] | CHENG Chunchun, LI Yulong, ZHANG Zhiqiang, LIU Xuejing. Study on dissolution crystallization for extraction of potassium and separation of magnesium and lithium from salt lake brine [J]. Inorganic Chemicals Industry, 2024, 56(6): 34-39. |
| [3] | HUANG Zhaojie, ZHAO Xiaoxu, WANG Haitao, CHANG Na, ZHANG Guoxin, XIE Yonglei, YIN Yanmei, LU Na, WANG Wei. Study on ultrafiltration+nanofiltration double membrane treatment of iron process iron phosphate production wastewater [J]. Inorganic Chemicals Industry, 2024, 56(11): 145-150. |
| [4] | HU Yue, MO Hengliang, LI Tianyu, PENG Wenjuan, SUN Guangdong, XIAO Hongkang, CHEN Yili, LI Suoding, TANG Yang. Study on spinning molding and performance of Ti-based lithium ion sieve powder and PVDF resin [J]. Inorganic Chemicals Industry, 2023, 55(11): 58-63. |
| [5] | LIN Yuqing,ZHANG Yiren,QIU Yulong,ZHANG Jiayu,YU Jianguo. Progress and prospect of membrane technology in lithium extraction from salt lake brine [J]. Inorganic Chemicals Industry, 2023, 55(1): 33-45. |
| [6] | ZHAO Zhanyi,ZHONG Yongheng,LIU Jia,LI Xiaoyan,YONG Meijing. Research on global patent distribution and development countermeasures of lithium industry [J]. Inorganic Chemicals Industry, 2022, 54(7): 10-17. |
| [7] | Liu Xianghuan,Yi Meigui,Xiang Lan. Preliminary study on separation of magnesium and lithium in multicomponent chloride salt system [J]. Inorganic Chemicals Industry, 2019, 51(8): 13-16. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||