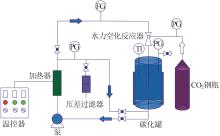
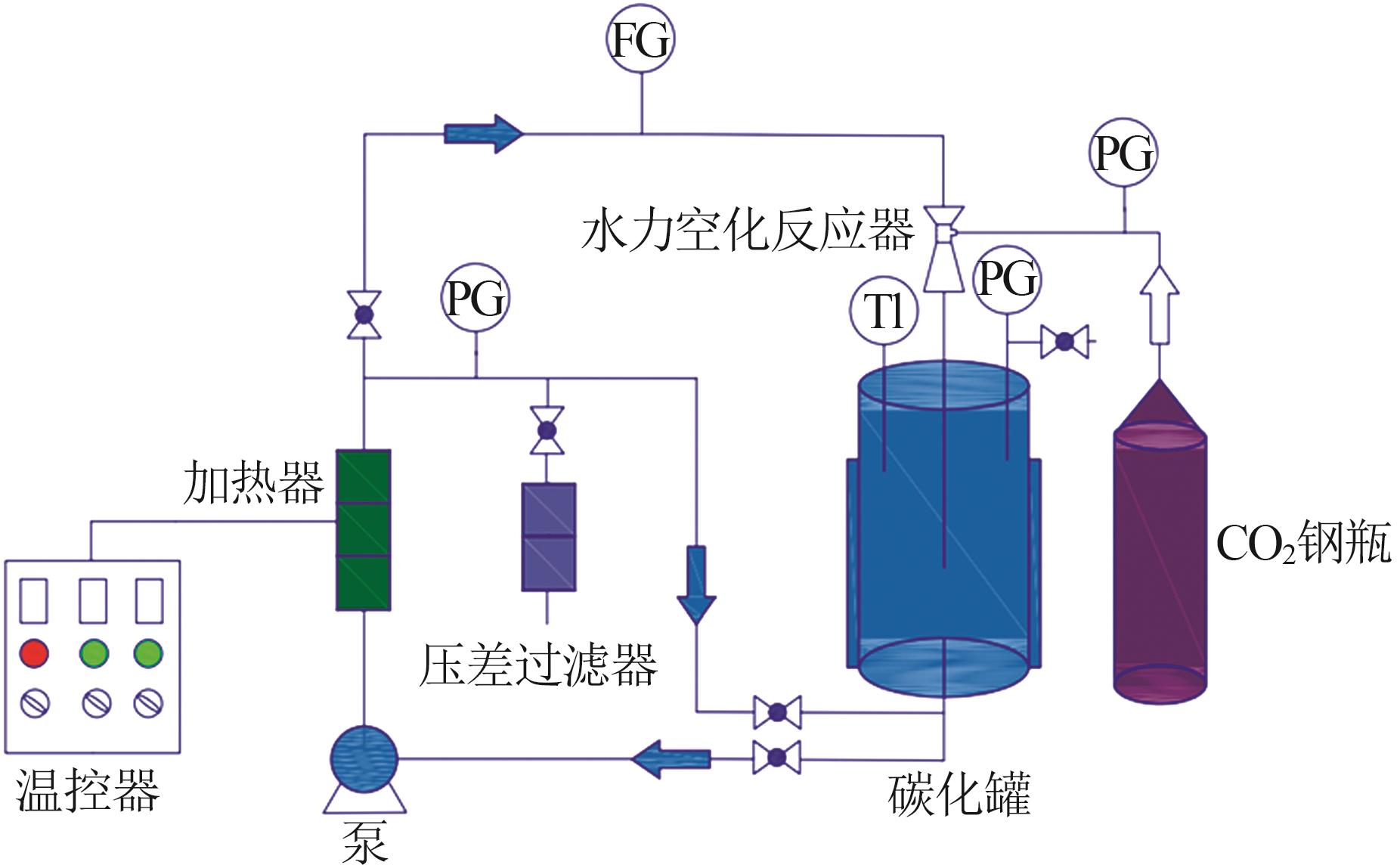
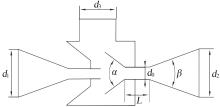
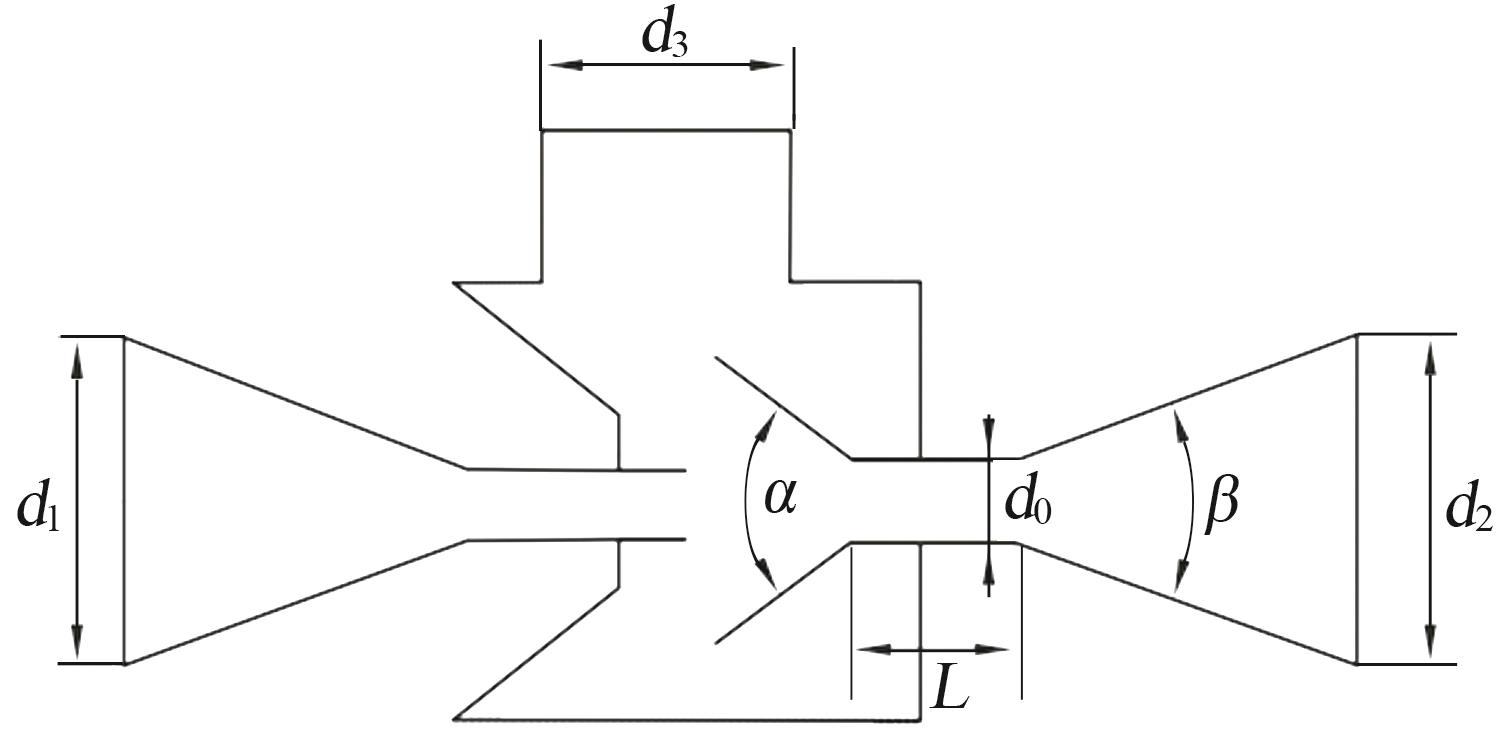
| 1 |
MA Xueyi, ZHAO Liang, WANG Dexi,et al.Numerical analysis of the gas⁃solid heat transfer characteristics of a lightly calcined MgO fluidized⁃bed roaster[J].Chemical Engineering Science,2023,267:118334.
|
| 2 |
张九磊,赵亮,董辉,等.轻烧氧化镁气流床煅烧炉热工过程及煅烧风量研究[J].无机盐工业,2022,54(1):96-100.
|
|
ZHANG Jiulei, ZHAO Liang, DONG Hui,et al.Study on the thermal process and calcination gas flow rate of light⁃burned magnesia entrained⁃flow calciner[J].Inorganic Chemicals Industry,2022, 54(1):96-100.
|
| 3 |
王瑜,李红,李雄伟,等.纳米氧化镁的生物制备及抗菌应用进展[J].现代化工,2021,41(8):58-62.
|
|
WANG Yu, LI Hong, LI Xiongwei,et al.Preparation of nano magnesium oxide through biological method and its application in antibacterial[J].Modern Chemical Industry,2021,41(8):58-62.
|
| 4 |
沈超,王丙垒,刘拼,等.氧化镁膨胀剂在某大体积混凝土工程中的应用[J].混凝土世界,2022(9):61-64.
|
|
SHEN Chao, WANG Binglei, LIU Pin,et al.Application of magnesium oxide expansive in a mass concrete project[J].China Concrete,2022(9):61-64.
|
| 5 |
PENG Lei, CHEN Bing.Study on the basic properties and mechanism of waste sludge solidified by magnesium phosphate cement containing different active magnesium oxide[J].Construction and Building Materials,2021,281:122609.
|
| 6 |
YU Honglei, WANG Dexi, LIU Niansheng,et al.Effect of modified nano/Mg(OH)2 on the flame retardancy and mechanical properties of NBR based on molecular simulation[J].Modelling and Simulation in Materials Science and Engineering,2022,30(5):055001.
|
| 7 |
YU Honglei, WANG Dexi, LI Yunlong,et al.Explainable molecular simulation and machine learning for carbon dioxide adsorption on magnesium oxide[J].Fuel,2024,357:129725.
|
| 8 |
PILARSKA A A, KLAPISZEWSKI Ł, JESIONOWSKI T.Recent development in the synthesis,modification and application of Mg(OH)2 and MgO:A review[J].Powder Technology,2017,319:373-407.
|
| 9 |
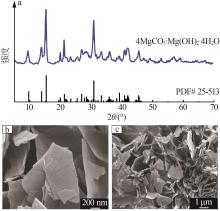
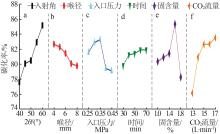
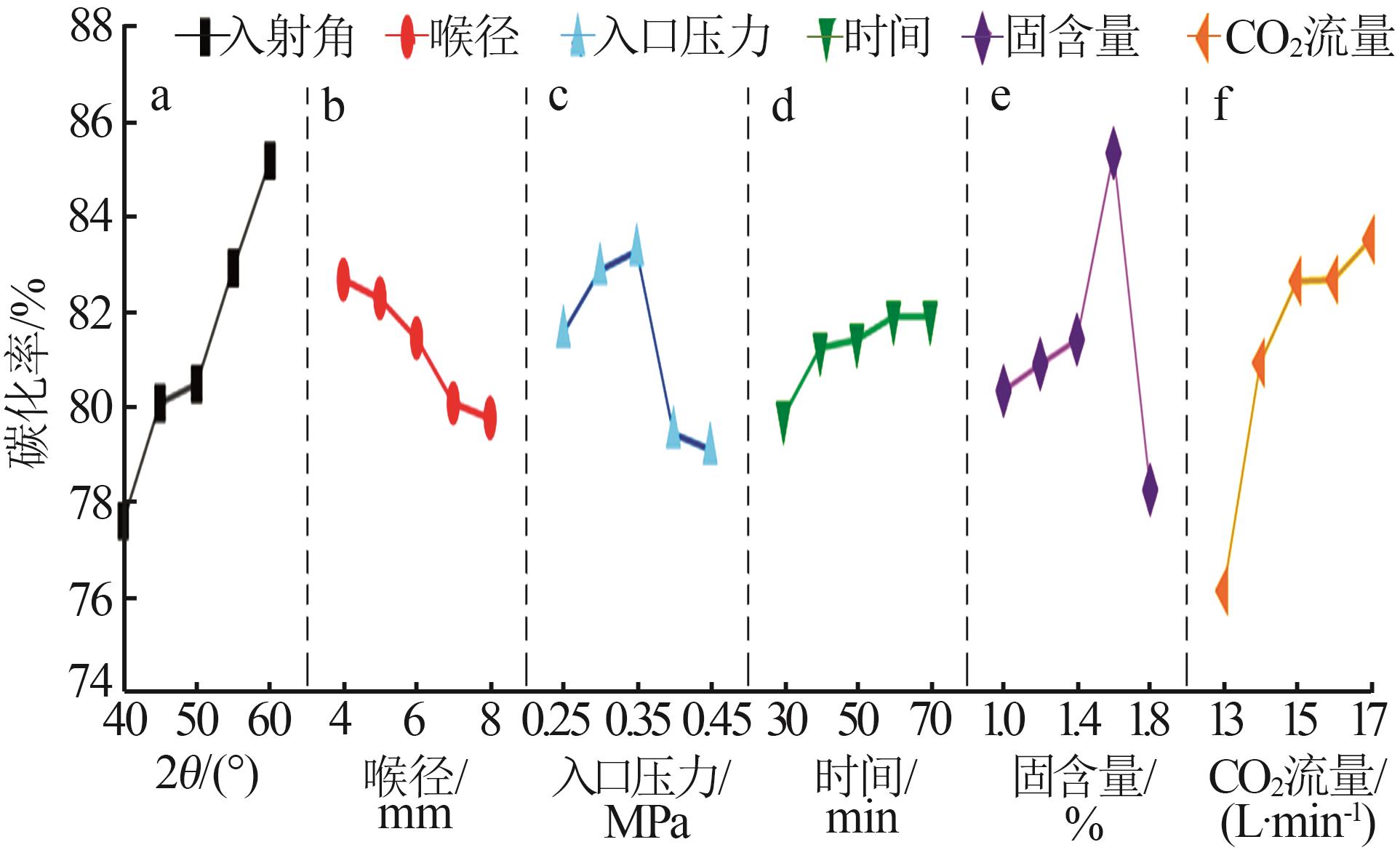
陈闵敏,孙玉柱,宋兴福,等.氢氧化镁碳化过程研究[J].华东理工大学学报(自然科学版),2022,48(5):600-608.
|
|
CHEN Minmin, SUN Yuzhu, SONG Xingfu,et al.Carbonization process of magnesium hydroxide[J].Journal of East China University of Science and Technology,2022,48(5):600-608.
|
| 10 |
刘家辉,宁志强,谢宏伟,等.氧化镁的制备方法及发展趋势综述[J].有色金属科学与工程,2021,12(3):12-19.
|
|
LIU Jiahui, NING Zhiqiang, XIE Hongwei,et al.Preparation methods and development trend of magnesium oxide[J].Nonferrous Metals Science and Engineering,2021,12(3):12-19.
|
| 11 |
张果泰,海春喜,周园.碱式碳酸镁的制备方法及应用现状[J].盐湖研究,2022,30(3):131-138.
|
|
ZHANG Guotai, Chunxi HAI, ZHOU Yuan.Preparation and application of basic magnesium carbonate[J].Journal of Salt Lake Research,2022,30(3):131-138.
|
| 12 |
刘淑杰,代淑娟,于秋月,等.低品位菱镁矿制备高纯氧化镁试验研究[J].非金属矿,2018,41(3):27-29.
|
|
LIU Shujie, DAI Shujuan, YU Qiuyue,et al.Experimental study on the preparation of high⁃purity magnesium oxide from low⁃grade magnesite[J].Non⁃Metallic Mines,2018,41(3):27-29.
|
| 13 |
王晓彤.菱镁矿碳化法制备高活性氧化镁[D].北京:北京化工大学,2016.
|
|
WANG Xiaotong.Preperation of highly active magnesium oxide by the carbonization of magnesite[D].Beijing:Beijing University of Chemical Technology,2016.
|
| 14 |
SMITHSON G L, BAKHSHI N N.Kinetics and mechanism of carbonation of magnesium oxide slurries[J].Industrial & Engineering Chemistry Process Design and Development,1973,12(1):99-106.
|
| 15 |
白俊红,刘有智,申红艳,等.超重力制备改性超细氢氧化镁的研究进展[J].无机盐工业,2014,46(5):10-12.
|
|
BAI Junhong, LIU Youzhi, SHEN Hongyan,et al.Research progress in preparation of modified ultrafine magnesium hydroxide with high gravity technology[J].Inorganic Chemicals Industry,2014,46(5):10-12.
|
| 16 |
杨卜,彭金辉,范兴祥,等.微波辐射煅烧碱式碳酸镁制备轻质活性氧化镁[J].无机盐工业,2005,37(11):26-28.
|
|
YANG Bo, PENG Jinhui, FAN Xingxiang,et al.The preparation of light and activated magnesium oxide from basic magnesium carbonate by microwave radiation⁃calcining[J].Inorganic Chemicals Industry,2005,37(11):26-28.
|
| 17 |
YAN Jingchen, AI Shuo, YANG Feng,et al.Study on mechanism of chitosan degradation with hydrodynamic cavitation[J].Ultrasonics Sonochemistry,2020,64:105046.
|
| 18 |
SAINTE BEUVE R, MORISON K R.Enzymatic hydrolysis of canola oil with hydrodynamic cavitation[J].Chemical Engineering and Processing:Process Intensification,2010,49(10):1101-1106.
|
| 19 |
BOKHARI A, CHUAH L F, YUSUP S,et al.Cleaner production of rubber seed oil methyl ester using a hydrodynamic cavitation:Optimisation and parametric study[J].Journal of Cleaner Production,2016,136:31-41.
|
| 20 |
BAXI P B, PANDIT A B.Using cavitation for delignification of wood[J].Bioresource Technology,2012,110:697-700.
|
 ), YU Honglei1, YANG Yali2, JIANG Jiuchuang2
), YU Honglei1, YANG Yali2, JIANG Jiuchuang2