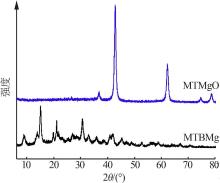
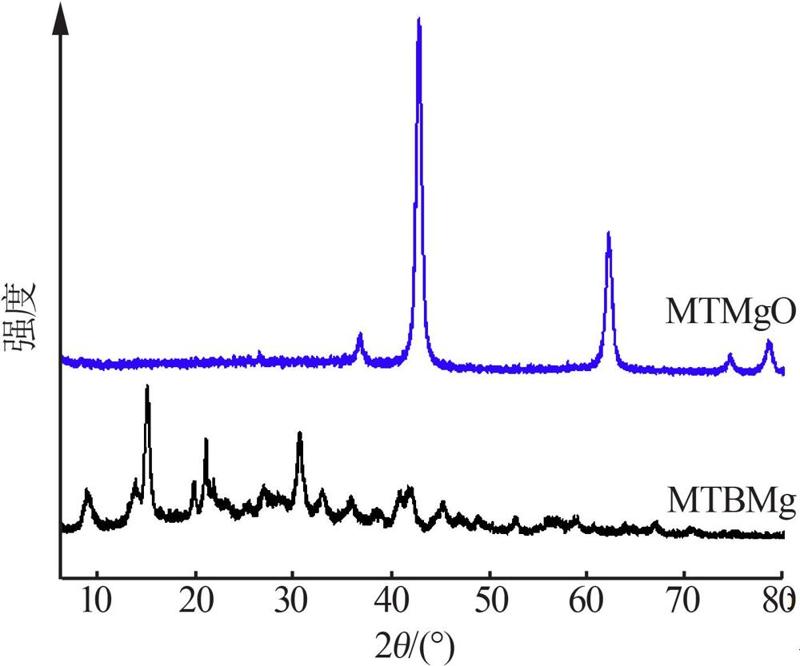
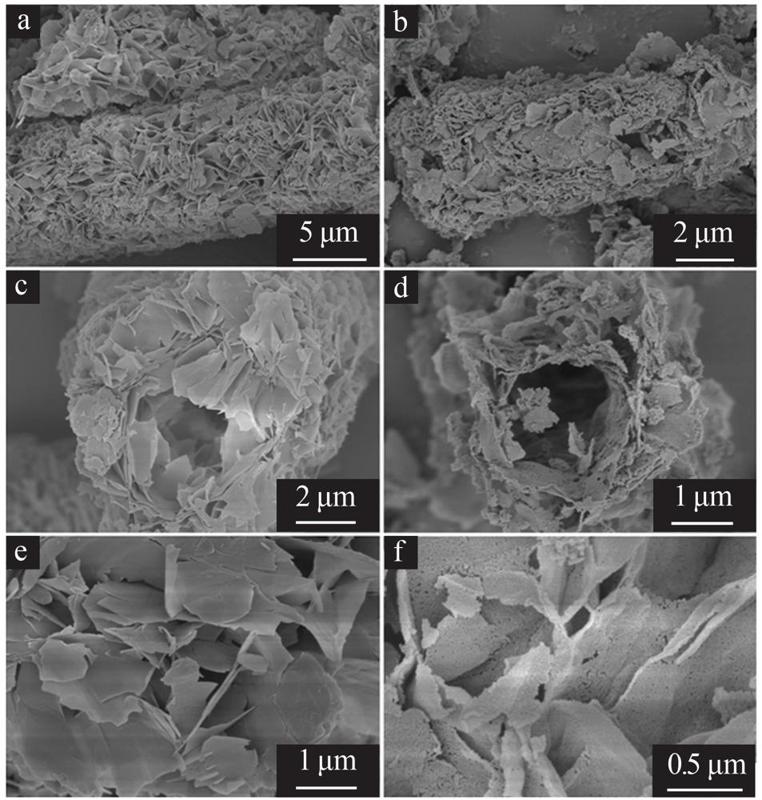
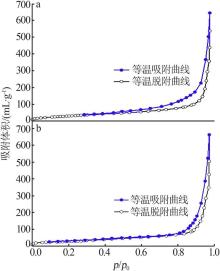
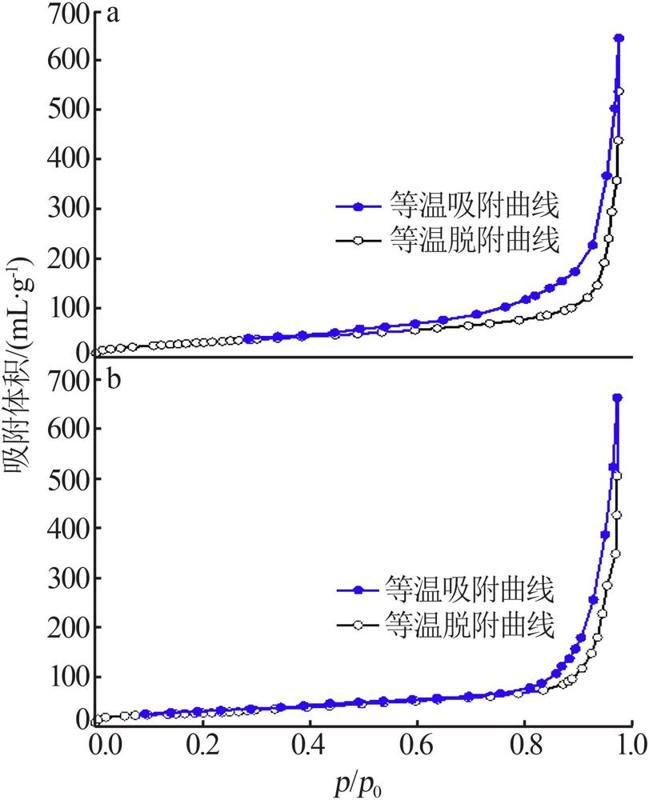
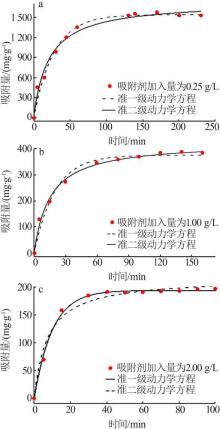
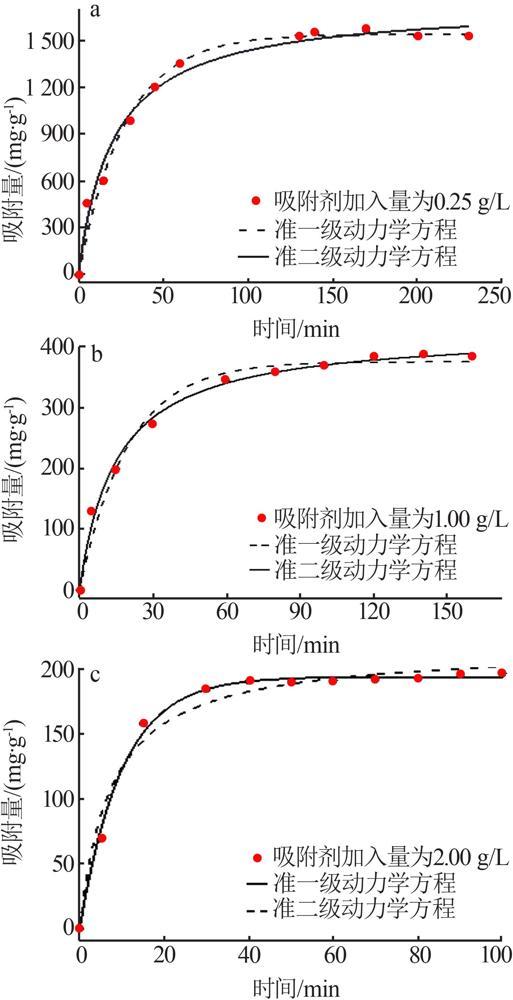
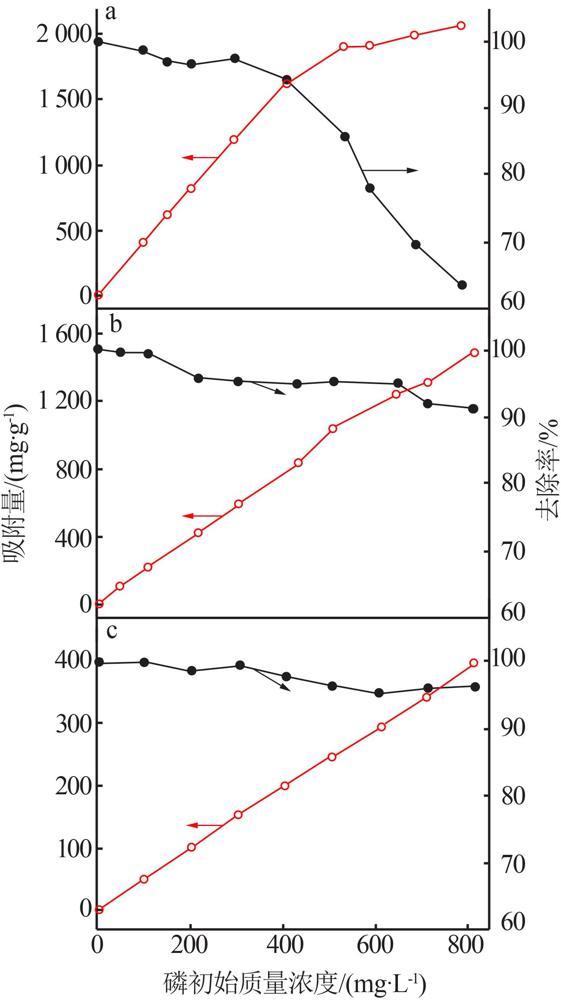
Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (9): 77-84.doi: 10.19964/j.issn.1006-4990.2021-0760
• Research & Development • Previous Articles Next Articles
Synthesis of micron?tubular?magnesium oxide clusters and its adsorption performance for phosphate
LIANG Hai1,2( ),YUAN Tianlong3(
),YUAN Tianlong3( ),WANG Wanting1,YANG Yunhong4,LIANG Wenjie1,WANG Xiaomin1,DENG Xinzhong1
),WANG Wanting1,YANG Yunhong4,LIANG Wenjie1,WANG Xiaomin1,DENG Xinzhong1
- 1. Liaoning Key Laboratory of Chemical Additive Synthesis and Separation,Yingkou Institute of Technology,Yingkou 115014,China
2. College of chemical engineering,Dalian Institute of Technology
3. College of mechanical and power Engineering,Yingkou Institute of Technology
4. Yingkou Magnesite Chemical Ind Group Co.,Ltd.
-
Received:2021-12-20Online:2022-09-10Published:2022-09-22 -
Contact:YUAN Tianlong E-mail:lianghai@yku.edu.cn;1584070637@163.com
CLC Number:
Cite this article
LIANG Hai,YUAN Tianlong,WANG Wanting,YANG Yunhong,LIANG Wenjie,WANG Xiaomin,DENG Xinzhong. Synthesis of micron?tubular?magnesium oxide clusters and its adsorption performance for phosphate[J]. Inorganic Chemicals Industry, 2022, 54(9): 77-84.
share this article
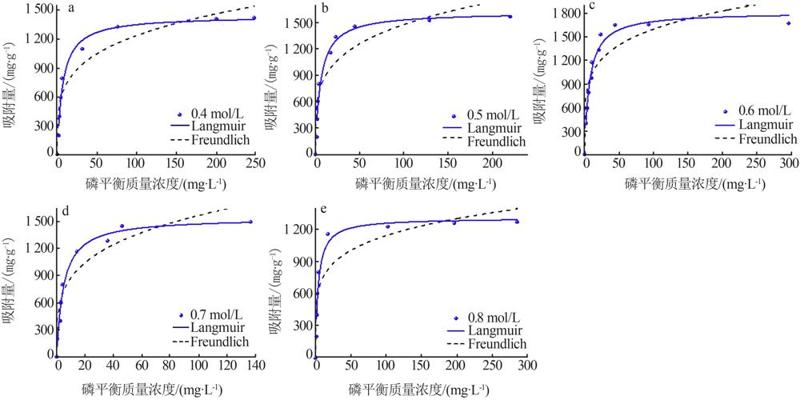
Table 2
Fitting parameters of isothermal adsorption of MTMgO synthesized with different concentrations of reactant"
c(Mg2+)/ (mol·L-1) | Langmuir模型 | Freundlich模型 | |||||
|---|---|---|---|---|---|---|---|
q/ (mg·g-1) | KL/ (L·mg-1) | R2 | n | KF/ (mg1-n ·L n ·g-1) | R2 | ||
| 0.4 | 1 441.83 | 0.15 | 0.97 | 4.15 | 408.80 | 0.91 | |
| 0.5 | 1 616.10 | 0.17 | 0.96 | 4.30 | 506.00 | 0.86 | |
| 0.6 | 1 810.85 | 0.14 | 0.96 | 5.10 | 649.00 | 0.81 | |
| 0.7 | 1 539.83 | 0.21 | 0.97 | 3.96 | 489.95 | 0.90 | |
| 0.8 | 1 303.28 | 0.26 | 0.95 | 5.34 | 481.90 | 0.85 | |
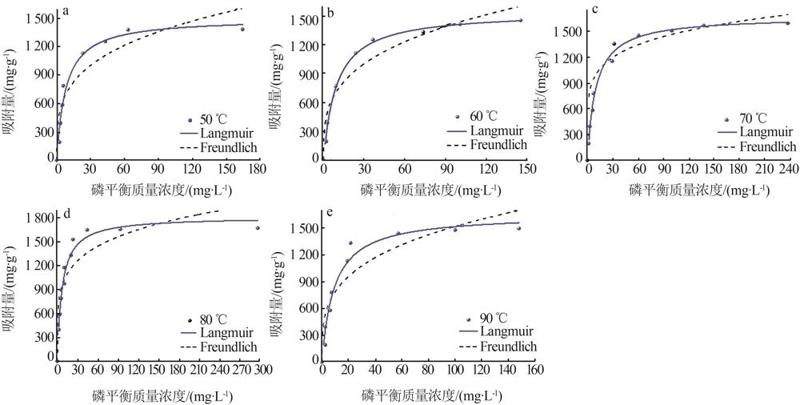
Table 3
Fitting parameters of isothermal adsorption of MTMgO synthesized at different hydrolysis temperatures"
| 水解温度/℃ | Langmuir模型 | Freundlich模型 | |||||
|---|---|---|---|---|---|---|---|
q/ (mg·g-1) | KL/ (L·mg-1) | R2 | n | KF/ (mg1-n ·L n ·g-1) | R2 | ||
| 50 | 1 503.6 | 0.12 | 0.96 | 3.62 | 393.03 | 0.86 | |
| 60 | 1 609.9 | 0.10 | 0.99 | 3.30 | 367.16 | 0.92 | |
| 70 | 1 661.4 | 0.10 | 0.99 | 5.97 | 678.40 | 0.86 | |
| 80 | 1 810.8 | 0.14 | 0.97 | 5.12 | 649.02 | 0.81 | |
| 90 | 1 656.3 | 0.10 | 0.96 | 3.51 | 408.95 | 0.85 | |
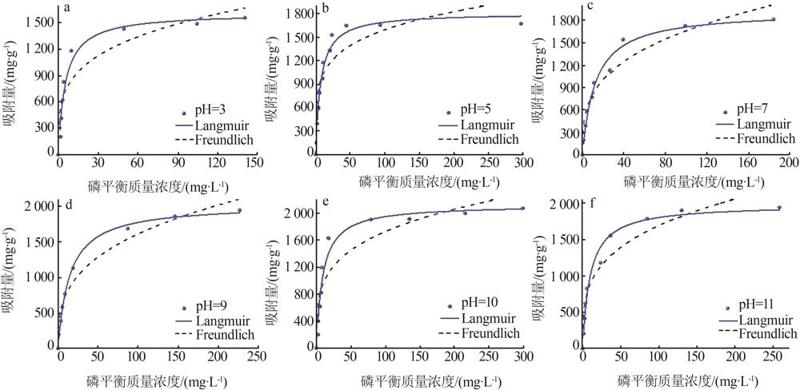
Table 4
Fitting parameters of isothermal adsorption ofMTMgO at different initial pH"
| pH | Langmuir模型 | Freundlich模型 | |||||
|---|---|---|---|---|---|---|---|
q/ (mg·g-1) | KL/ (L·mg-1) | R2 | KF/ (mg1-n ·L n ·g-1) | n | R2 | ||
| 3 | 1 615.52 | 0.19 | 0.94 | 474.76 | 3.93 | 0.86 | |
| 5 | 1 810.85 | 0.14 | 0.96 | 649.02 | 5.12 | 0.81 | |
| 7 | 1 922.37 | 0.08 | 0.98 | 396.91 | 3.22 | 0.93 | |
| 9 | 2 034.37 | 0.07 | 0.99 | 363.07 | 3.08 | 0.96 | |
| 10 | 2 120.12 | 0.11 | 0.95 | 539.95 | 3.96 | 0.85 | |
| 11 | 1 975.92 | 0.10 | 0.97 | 463.01 | 3.56 | 0.92 | |
| [1] |
SHAKOOR M B, YE Zhilong, CHEN Shaohua.Engineered biochars for recovering phosphate and ammonium from wastewater:A review[J].Science of the Total Environment,2021,779.Doi:10.1016/j.scitotenv.2021.
doi: 10.1016/j.scitotenv.2021. |
| [2] | FANG Liping, WU Baile, CHAN J K M, et al.Lanthanum oxide nanorods for enhanced phosphate removal from sewage:A response surface methodology study[J].Chemosphere,2018,192:209-216. |
| [3] | MORELLIB, HAWKINST R,NIBLICKB,et al.Critical review of eutrophication models for life cycle assessment[J].Environmental Sci ence & Technology,2018,52(17):9562-9578. |
| [4] |
WEI Ting, LI Qiangang, WANG Hongjie, et al.Advanced phosphate and nitrogen removal in water by La-Mg composite[J].Environmental Research,2021,193.Doi:10.1016/j.envres.2020.110529.
doi: 10.1016/j.envres.2020.110529. |
| [5] |
ZHU Danchen, YANG Haiping, CHEN Xu, et al.Temperature⁃dependent magnesium citrate modified formation of MgO nanoparticles biochar composites with efficient phosphate removal[J].Che⁃ mosphere,2021,274.Doi:10.1016/j.chemosphere.2021.129904.
doi: 10.1016/j.chemosphere.2021.129904. |
| [6] |
ZHAO Chenxi, WANG Bing, THENG B K G, et al.Formation and mechanisms of nano⁃metal oxide⁃biochar composites for pollutants removal:A review[J].Science of the Total Environment,2021,767. Doi:10.1016/j.scitotenv.2021.145305.
doi: 10.1016/j.scitotenv.2021.145305. |
| [7] | GHAEDI A M, VAFAEI A.Applications of artificial neural networks for adsorption removal of dyes from aqueous solution:A review[J].Advances in Colloid and Interface Science,2017,245:20-39. |
| [8] | GHAEDI A M, PANAHIMEHR M, NEJAD A R S, et al.Factorial experimental design for the optimization of highly selective adsorption removal of lead and copper ions using metal organic framework MOF-2(Cd)[J].Journal of Molecular Liquids,2018,272:15-26. |
| [9] |
LUO Haoyu, WANG Yijie, WEN Xiaoqing, et al.Key roles of the crystal structures of MgO-biochar nanocomposites for enhancing phosphate adsorption[J].Science of the Total Environment,2021,766. Doi:10.1016/j.scitotenv.2020.142618.
doi: 10.1016/j.scitotenv.2020.142618. |
| [10] | GUO Wanli, NAZIM H, LIANG Zongsuo, et al.Magnesium deficiency in plants:An urgent problem[J].The Crop Journal,2016,4(2):83-91. |
| [11] |
YE Tiantian, MIN Xiangyu, LI Xinju, et al.Improved holding and releasing capacities of coal gangue toward phosphate through alkali⁃activation[J].Chemosphere,2022,287.Doi:10.1016/j.chemosphere.2021.32382.
doi: 10.1016/j.chemosphere.2021.32382. |
| [12] |
XI Huan, JIANG Haoli, ZHAO Dan, et al.Highly selective adsorption of phosphate from high⁃salinity water environment using MgO-loaded and sodium alginate⁃immobilized bentonite beads[J].Journal of Cleaner Production,2021,313.Doi:10.1016/j.jclepro. 2021.127773.
doi: 10.1016/j.jclepro. 2021.127773. |
| [13] |
XIA Yan, DONG Kangyu, XIANG Xiangmei, et al.Phosphorus hyperaccumulation in nano-MgO using a circular recovery process based on multiple phase transitions from periclase to bruci⁃te[J].Science of the Total Environment,2020,727.Doi:10.1016/j.scitotenv.2020.138510.
doi: 10.1016/j.scitotenv.2020.138510. |
| [14] | 杨斌,苏琪,杨高玲,等.喷雾干燥法制备氧化镁材料及其吸附性能[J].化工学报,2018,69(7):3068-3075. |
| YANG Bin, SU Qi, YANG Gaoling, et al.Preparation and adsorption properties of magnesium oxide via spray drying[J].CIESC Journal,2018,69(7):3068-3075. | |
| [15] | YU Zhichao, XU Chonghe, YUAN Kangkang, et al.Template-free synthesis of MgO mesoporous nanofibers with superior adsorption for fluoride and Congo red[J].Ceramics International,2018,44(8):9454-9462. |
| [16] | 郑力.氧化镁/果胶复合除磷剂的制备及其除磷机理研究[D].武汉:华中科技大学,2017. |
| ZHENG Li.Identification of the mechanism for phosphates removal by using A modified phosphate absorbent prepared from MgO and pectin[D].Wuhan:Huazhong University of Science and Technology,2017. | |
| [17] | 葛立发.纳米氧化镁复合材料的制备与性能研究[D].武汉:华中科技大学,2019. |
| GE Lifa.Investigation on preparation of magnesium oxide nanocompositesand their properties[D].Wuhan:Huazhong University of Science and Technology,2019. | |
| [18] | WANG Tao, XU Youfeng, SU Qiying, et al.Hierarchical porous nanosheet⁃assembled MgO microrods with high adsorption capacity[J].Materials Letters,2014,116:332-336. |
| [19] | JIN Zhen, JIA Yong, ZHANG Kaisheng, et al.Effective removal of fluoride by porous MgO nanoplates and its adsorption mechanism[J].Journal of Alloys and Compounds,2016,675:292-300. |
| [20] | 张雅君.小分子自组装形成纳米管的机理研究[D].苏州:苏州大学,2017. |
| ZHANG Yajun.Formation mechanism of nanotubes via self⁃assembly of a small molecule[D].Suzhou:Soochow University,2017. | |
| [21] | 覃利琴,杨黄根,陈德,等.辅助溶剂热法合成纳米晶自组装CaWO4:Tb3+微球及其发光性能[J].无机盐工业,2021,53(1):36-39,43. |
| QIN Liqin, YANG Huanggen, CHEN De, et al.Assistanted solvothermal synthesis of nanocrystalline self⁃assembled CaWO4:Tb3+ microspheres and luminescence properties[J].Inorganic Chemicals Industry,2021,53(1):36-39,43. | |
| [22] |
LIU Xiaoning, FU Junyan, TANG Yiwei, et al.Mg-coordinated self-assembly of MgO-doped ordered mesoporous carbons for selective recovery of phosphorus from aqueous solutions[J].Chemical Engineering Journal,2021,406.Doi:10.1016/j.cej.2020.126748.
doi: 10.1016/j.cej.2020.126748. |
| [23] | 常军,贾福康,胡成山,等.电解锰渣基沸石对锰离子的吸附性能研究[J].无机盐工业,2019,51(9):61-66. |
| CHANG Jun, JIA Fukang, HU Chengshan, et al.Adsorption of manganese ion by zeolite synthesized from electrolytic manganese residue[J].Inorganic Chemicals Industry,2019,51(9):61-66. | |
| [24] |
LIU Jiwei, JIANG Jianguo, AIHEMAITI A, et al.Removal of phosphate from aqueous solution using MgO-modified magnetic biochar derived from anaerobic digestion residue[J].Journal of Environmental Management,2019,250.Doi:10.1016/j.jenvman.2019.109438.
doi: 10.1016/j.jenvman.2019.109438. |
| [25] | XIA Peng, WANG Xuejing, WANG Xin, et al.Struvite crystallization combined adsorption of phosphate and ammonium from aqueous solutions by mesoporous MgO loaded diatomite[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2016,506:220-227. |
| [26] | 李友凤,敖克厚,樊磊磊,等.氧化铈-二氧化硅介孔材料制备及对铜离子的吸附性能[J].无机盐工业,2020,52(12):34-39. |
| LI Youfeng, AO Kehou, FAN Leilei, et al.Preparation of CeO2-SiO2 mesoporous material and its adsorption property for copper ions[J].Inorganic Chemicals Industry,2020,52(12):34-39. |
| [1] | DONG Nan, WANG Nan, JI Lijun, SHENG Yong. Determination of potassium salt solubility at low temperature and study of liquid fertilizer formula [J]. Inorganic Chemicals Industry, 2025, 57(2): 92-97. |
| [2] | ZHOU Wanji, LI Sixia. Study on extraction lithium from brine with high magnesium using ionic liquid/metal salt extraction system [J]. Inorganic Chemicals Industry, 2024, 56(9): 54-59. |
| [3] | ZHAO Leilei, SHU Yizhou, QI Zenglian, LIU Dandan, KUANG Jialing, HUANG Chengdong, WU Jiaxiang, PU Zhengxian. Effect of different addition amount of ammonium polyphosphate on granulation performance of compound fertilizer [J]. Inorganic Chemicals Industry, 2024, 56(8): 74-82. |
| [4] | SUN Lan, CHEN Shiying, YANG Liuxu, NIU Yiming, ZHAO Aonan. Basic study on fixing soluble phosphorus in phosphogypsum with magnesium slag [J]. Inorganic Chemicals Industry, 2024, 56(8): 92-98. |
| [5] | WANG Wei, LI Wei, LI Limin, LIU Dongxu. Study on pretreatment of iron phosphate production wastewater reuse and zero discharge [J]. Inorganic Chemicals Industry, 2024, 56(8): 99-103. |
| [6] | SU Baocai, ZHANG Qin, XIE Yuanjian, CAI Pingxiong, PAN Yuanfeng. Advances in synthesis methods and structural modification of LiMnFePO4 materials [J]. Inorganic Chemicals Industry, 2024, 56(7): 28-36. |
| [7] | ZHANG Lijin, LÜ Qing, CHEN Xiaolang, LI Qingxin, SHI Hongyu, QIN Jun. Preparation of Ca-based LDO composite material and its adsorption performance for phosphate [J]. Inorganic Chemicals Industry, 2024, 56(7): 37-45. |
| [8] | YANG Hanshuo, WANG Dexi, YU Honglei, YANG Yali, JIANG Jiuchuang. Experimental study on hydrodynamic cavitation⁃enhanced carbothermic reduction process for production of magnesium carbonate hydromagnesite [J]. Inorganic Chemicals Industry, 2024, 56(7): 74-79. |
| [9] | WANG Junting, MA Hang, ZHA Zuotong, WAN Banglong, ZHANG Zhenhuan. Research progress of iron phosphate industrial wastewater treatment process [J]. Inorganic Chemicals Industry, 2024, 56(6): 26-33. |
| [10] | LI Yongxiang, YANG Xiaolong, LU Zhonghai, MA Hang, WAN Banglong, LI Yundong. Research progress of flame retardant application of ammonium poly-phosphate [J]. Inorganic Chemicals Industry, 2024, 56(5): 20-30. |
| [11] | XU Mengyao, ZHANG Xin, HE Kunpeng, HE Jian, JIANG Wei. Preparation of yttrium oxide and zirconium phosphate adsorbents from zirconium-yttrium waste and evaluation of their performance [J]. Inorganic Chemicals Industry, 2024, 56(3): 116-124. |
| [12] | WANG Jianrui, ZHANG Song, ZHANG Jie. Study on new process for beneficiation phosphate of low-grade phosphate rock leaching via lactic acid [J]. Inorganic Chemicals Industry, 2024, 56(3): 56-63. |
| [13] | LU Yunkun, TANG Xianyou, YIN Hang, ZHANG Yanan, ZHANG Shaojie. Study on surface packaging and leakage prevention of high temperature molten salt/ceramic composite phase change thermal storage materials [J]. Inorganic Chemicals Industry, 2024, 56(2): 80-85. |
| [14] | XU Miao, XU Dehua, SHOU Zhixin, YANG Wengong, WANG Xinlong. Study on preparation process of magnesium ammonium polyphosphate [J]. Inorganic Chemicals Industry, 2024, 56(12): 88-93. |
| [15] | ZHAO Runze, QIAN A′niu. Research progress of lithium recovery for spent lithium-ion batteries and preparation in battery-grade lithium carbonate [J]. Inorganic Chemicals Industry, 2024, 56(12): 70-78. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||