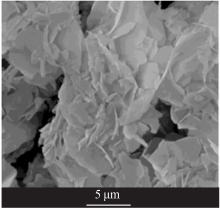
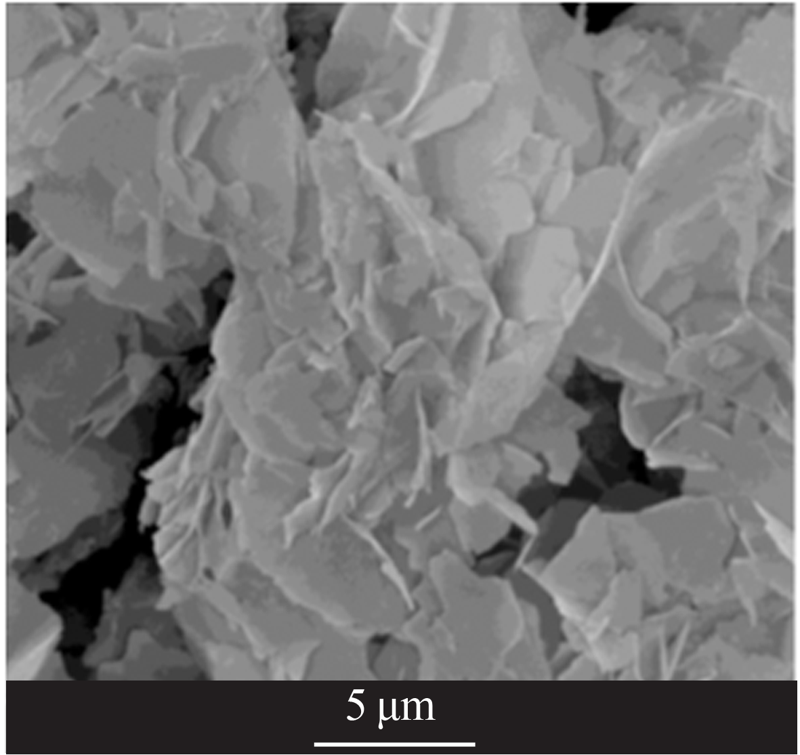
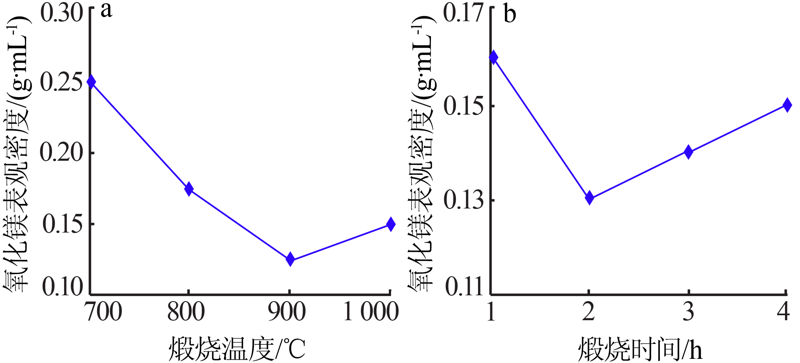
| [1] |
宋达, 王俐聪, 吴丹, 等. 以卤水为原料制备高纯氧化镁研究进展[J]. 盐科学与化工, 2018, 47(5):8-11.
|
| [2] |
郭如新. 镁资源、镁质化工材料现状与前景[J]. 无机盐工业, 2012, 44(10):1-7.
|
| [3] |
张浩波, 刘建路, 何加海, 等. 以卤水为原料生产氧化镁的技术研究[J]. 盐科学与化工, 2018, 47(10):36-40.
|
| [4] |
张华, 聂鹏飞, 徐春和, 等. 白云石制备高纯氧化镁的工艺研究[J]. 无机盐工业, 2012, 44(4):22-24.
|
| [5] |
徐徽, 蔡勇, 石西昌, 等. 水镁石制取高纯氧化镁的研究[J]. 湖南师范大学自然科学学报, 2006, 29(1):52-55.
|
| [6] |
陈春英, 周佳芬, 路贵民, 等. 高纯镁砂及氧化镁陶瓷研究进展[J]. 化工进展, 2019, 38(1):505-515.
|
| [7] |
邓绍雄. 高纯氧化镁生产的历史回顾与展望[J]. 耐火材料, 1994, 28(5):289-292.
|
| [8] |
陈峥, 刘素芹, 张金娜. 高纯氧化镁的研究与开发[J]. 盐科学与化工, 2017, 46(4):32-35.
|
| [9] |
张浩波, 何加海, 袁敬明, 等. 一种制备高纯氧化镁的方法:中国,109354047A[P]. 2019-02-19.
|
| [10] |
中昊(大连)化工研究设计院有限公司. 纯碱工学[M].3版. 北京: 化学工业出版社, 2014.
|
| [11] |
陈娟. 碱式碳酸镁晶形控制及其生长机理研究[D]. 武汉:武汉工程大学, 2017.
|
| [12] |
杨晨. 多晶相水合碳酸镁结晶生长过程调控研究[D]. 上海:华东理工大学, 2013.
|
| [13] |
Kloprogge J, Martens W, Nothdurft L, et al. Low temperature synjournal and characterization of nesquehonite[J]. Journal of Materials Science Letters, 2003, 22(11):825-829.
doi: 10.1023/A:1023916326626
|
| [14] |
张士航. 由制盐母液制取碱式碳酸镁和氧化镁[J]. 山东化工, 2017, 46(15):23-24,27.
|
| [15] |
Hopkinson L, Rutt K, Cressey G. The transformation of nesqueho-nite to hydromagnesite in the system CaO-MgO-H2O-CO2:An ex-perimental spectroscopic study[J]. The Journal of Geology, 2008, 106(4):387-400.
|
| [16] |
张向京, 赵飒, 张志昆, 等. 加压碳化法制备碱式碳酸镁新工艺研究[J]. 无机盐工业, 2011, 43(10):39-41.
|
 ),Zhang Haobo3,Wu Zhongping3,Huang Huilin3
),Zhang Haobo3,Wu Zhongping3,Huang Huilin3