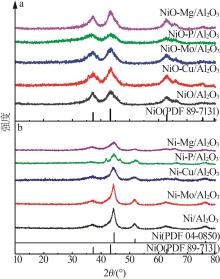
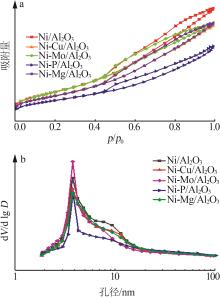
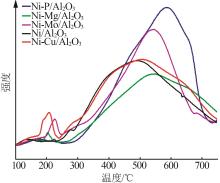
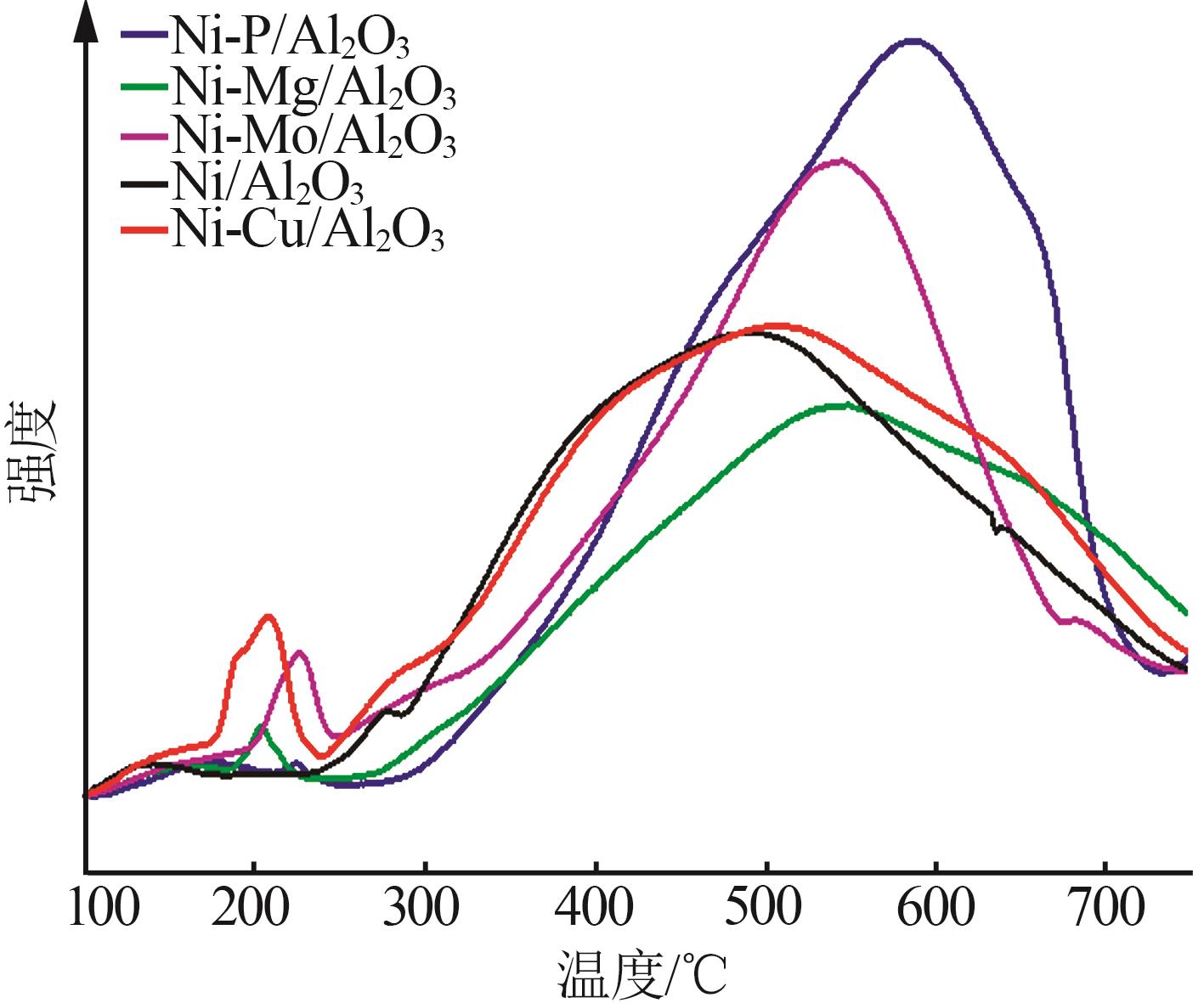
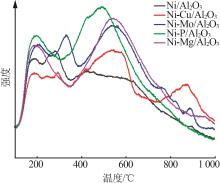
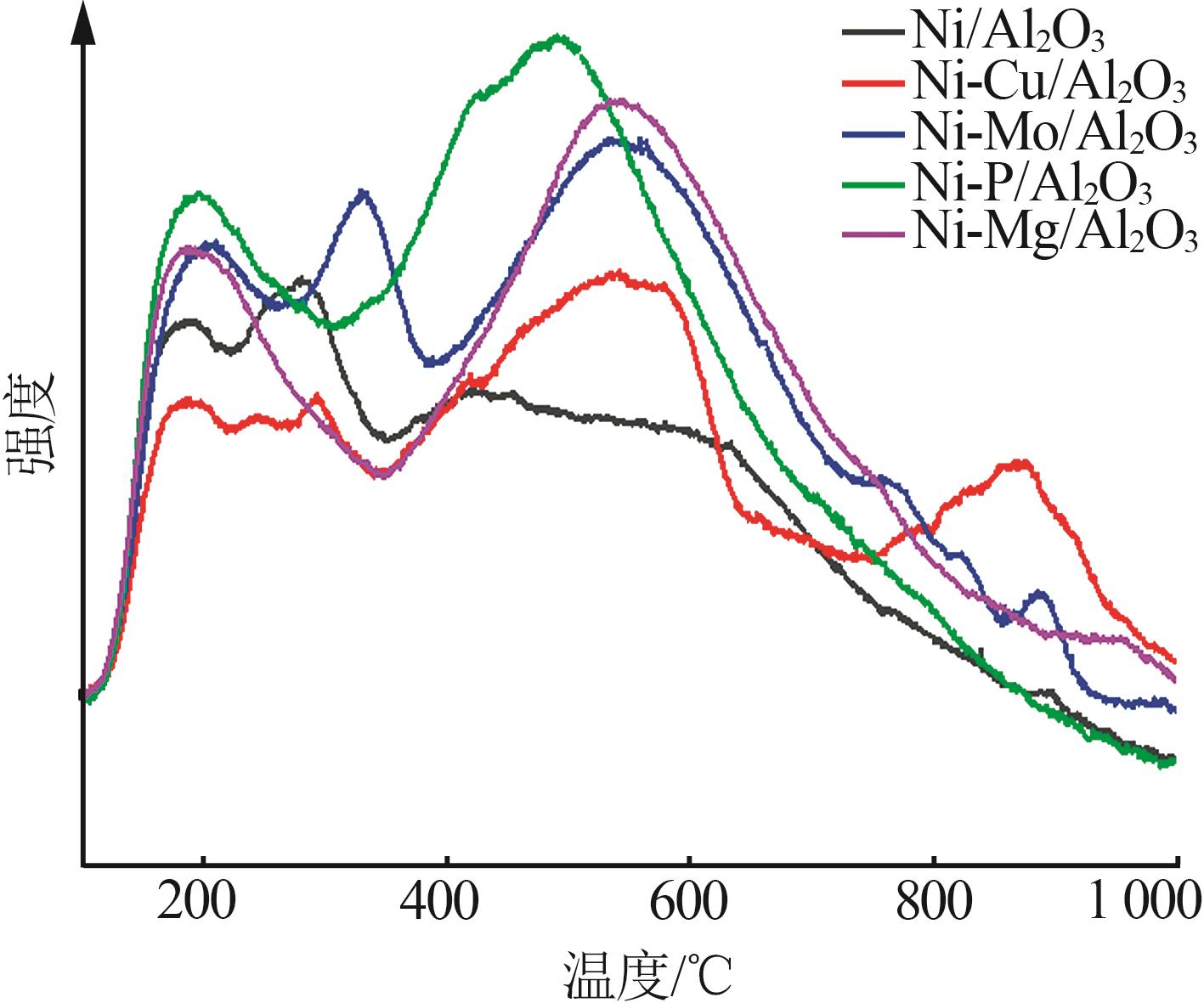
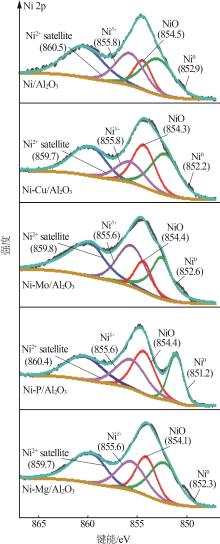
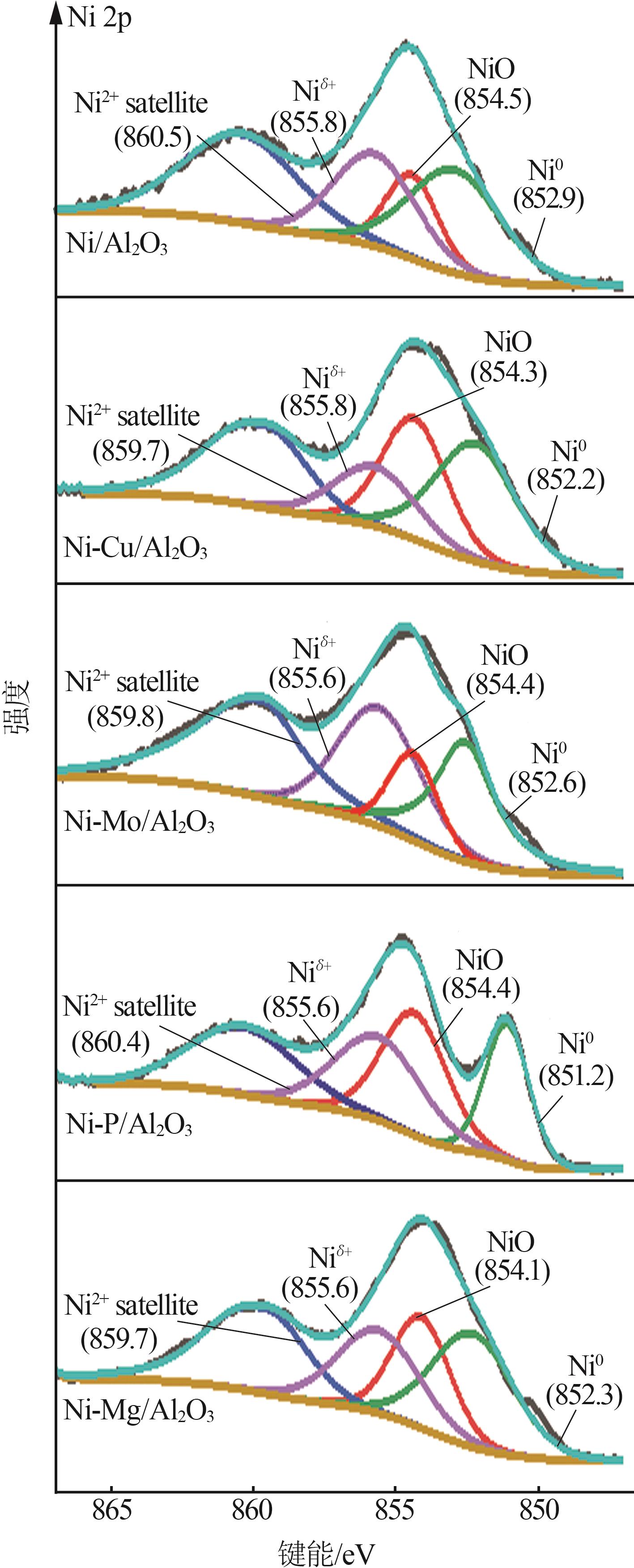
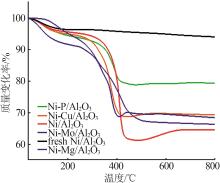
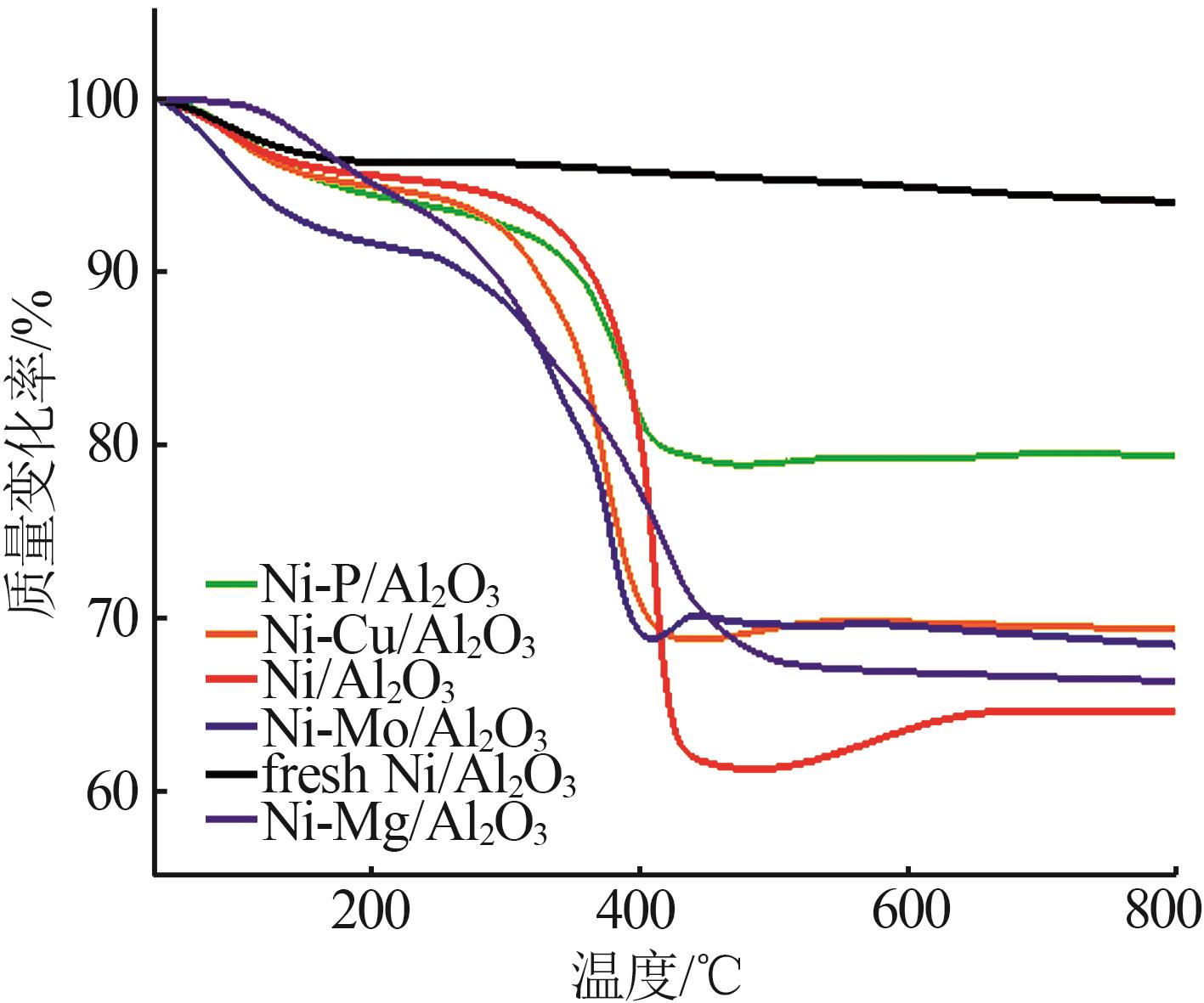
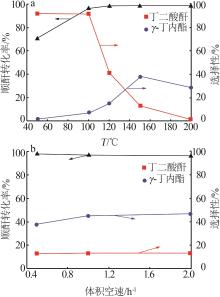
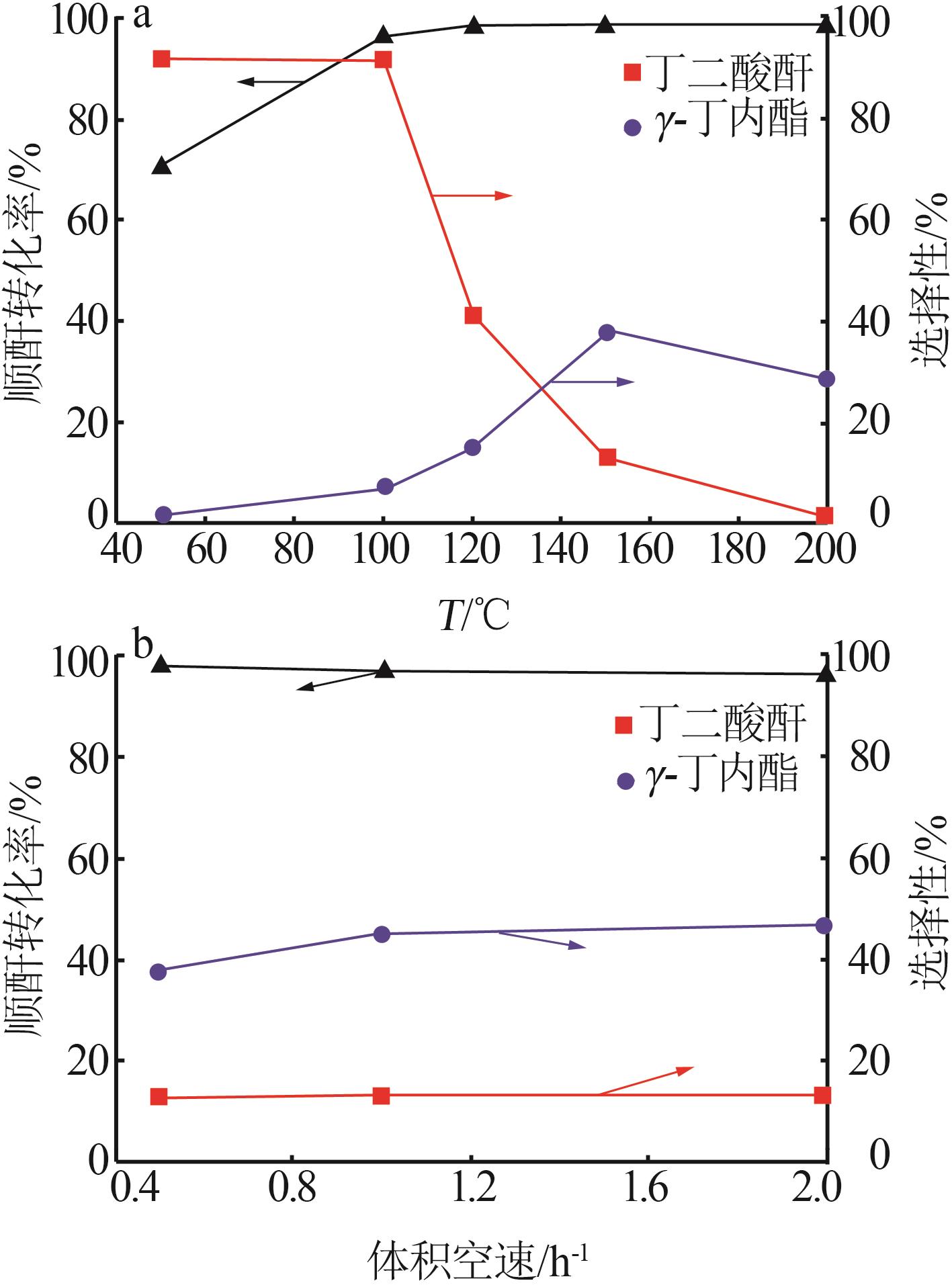
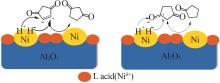
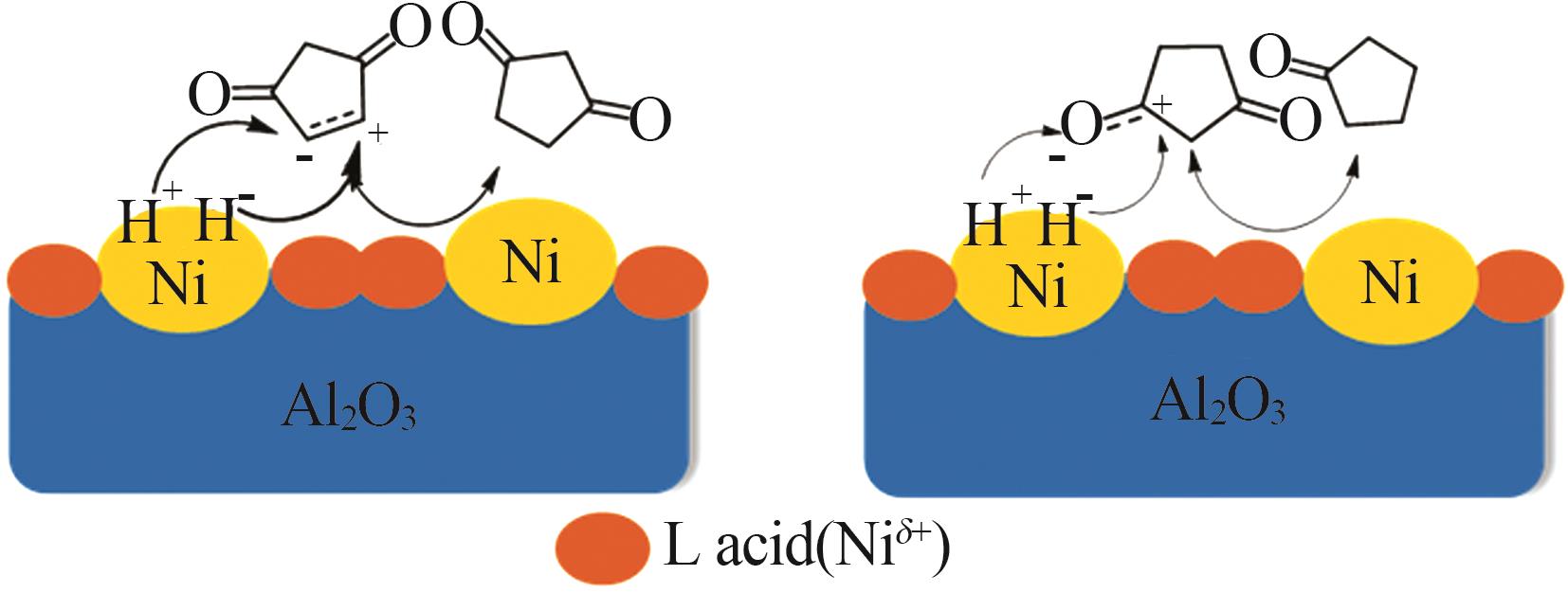
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (4): 143-150.doi: 10.19964/j.issn.1006-4990.2023-0417
• Catalytic Materials • Previous Articles
Effect of different additives on liquid phase selective hydrogenation performance of maleic anhydride over Ni/Al2O3
ZHU Jinjian1( ), YANG Xiaxia2, MU Zhanpeng2, SONG Guoliang1, LI Zihan1, LIU Wei1, XU Yan1, ZHANG Jingcheng1
), YANG Xiaxia2, MU Zhanpeng2, SONG Guoliang1, LI Zihan1, LIU Wei1, XU Yan1, ZHANG Jingcheng1
- 1.CNOOC Tianjin Chemical Research and Design Institute Co., Ltd., Tianjin 300131, China
2.China University of Petroleum, Beijing 102249, China
-
Received:2023-08-18Online:2024-04-10Published:2024-04-18
CLC Number:
Cite this article
ZHU Jinjian, YANG Xiaxia, MU Zhanpeng, SONG Guoliang, LI Zihan, LIU Wei, XU Yan, ZHANG Jingcheng. Effect of different additives on liquid phase selective hydrogenation performance of maleic anhydride over Ni/Al2O3[J]. Inorganic Chemicals Industry, 2024, 56(4): 143-150.
share this article
| 1 |
SOLSONA V, MORALES-DE LA ROSA S, DE LUCA O, et al. Solvent additive-induced deactivation of the Cu-ZnO(Al2O3)-catalyzed γ-butyrolactone hydrogenolysis:A rare deactivation proce-ss[J]. Industrial & Engineering Chemistry Research, 2021, 60(44):15999-16010.
doi: 10.1021/acs.iecr.1c04080 |
| 2 | ZHOU Yafen, WANG Qing, WANG Manman, et al. Promotional effect of CoO(OH) on selective hydrogenation of maleic anhydride to γ-butyrolactone over supported ruthenium catalyst[J]. China Petroleum Processing & Petrochemical Technology, 2015, 17(4):96-101. |
| 3 | 梁斌, 李宝岩, 金荣华, 等. γ-丁内酯的工业应用进展[J]. 上海师范大学学报(自然科学版), 2021, 50(5):629-635. |
| LIANG Bin, LI Baoyan, JIN Ronghua, et al. Progress in industrial application of γ-butyrolactone[J]. Journal of Shanghai Normal University(Natural Sciences), 2021, 50(5):629-635. | |
| 4 | 何仕明, 王俊文, 张玮, 等. 苯氧化法制顺酐连续化生产工艺设计及评价[J]. 化学工程, 2022, 50(4):73-78. |
| HE Shiming, WANG Junwen, ZHANG Wei, et al. Process design and evaluation of continuous production of maleic anhydride by benzene oxidation[J]. Chemical Engineering(China), 2022, 50(4):73-78. | |
| 5 |
FAIZAN M, LI Yingwei, ZHANG Ruirui, et al. Progress of vanadium phosphorous oxide catalyst for n-butane selective oxidati-on[J]. Chinese Journal of Chemical Engineering, 2022, 43:297- 315.
doi: 10.1016/j.cjche.2021.10.026 |
| 6 |
MÜLLER M, KUTSCHERAUER M, BÖCKLEIN S, et al. On the importance of by-products in the kinetics of n-butane oxidation to maleic anhydride[J]. Chemical Engineering Journal, 2020, 401:126016.
doi: 10.1016/j.cej.2020.126016 |
| 7 | 肖刚. 国内顺酐现状及发展趋势[J]. 聚酯工业, 2023, 36(3):13-17. |
| XIAO Gang. The current situation and development trend of Maleic anhydride in China[J]. Polyester Industry, 2023, 36(3):13-17. | |
| 8 |
BAI Yang, CHERKASOV N, HUBAND S, et al. Highly selective continuous flow hydrogenation of cinnamaldehyde to cinnamyl alcohol in a Pt/SiO2 coated tube reactor[J]. Catalysts, 2018, 8(2): 58.
doi: 10.3390/catal8020058 |
| 9 | WANG Jiandian, SUN Changyong, XIA Wenjun, et al. Pd/BN catalysts for highly efficient hydrogenation of maleic anhydride to succinic anhydride[J]. Applied Catalysis A:General, 2022, 630:118471. |
| 10 |
LI Haoyu, WU Pengfei, LI Xianquan, et al. Catalytic hydrogenation of maleic anhydride to γ-butyrolactone over a high-performance hierarchical Ni-Zr-MFI catalyst[J]. Journal of Catalysis, 2022, 410:69-83.
doi: 10.1016/j.jcat.2022.04.011 |
| 11 |
ZHAO Lili, ZHAO Jianghong, WU Tianjie, et al. Synergistic effect of oxygen vacancies and Ni species on tuning selectivity of Ni/ZrO₂ catalyst for hydrogenation of maleic anhydride into succinic anhydride and γ-butyrolacetone[J]. Nanomaterials, 2019, 9(3):406.
doi: 10.3390/nano9030406 |
| 12 |
YU Zhiquan, WANG Anjie, LIU Shan, et al. Hydrodeoxygenation of phenolic compounds to cycloalkanes over supported nickel phosphides[J]. Catalysis Today, 2019, 319:48-56.
doi: 10.1016/j.cattod.2018.05.012 |
| 13 | ZHOU Yafen, CHEN Qilin, WANG Qing, et al. Selective hydrogenation of maleic anhydride to succinic anhydride over nickel catalyst supported on carbon microspheres[J]. China Petroleum Processing & Petrochemical Technology, 2021, 23(4):75-82. |
| 14 |
REDDY KANNAPU H P, MULLEN C A, ELKASABI Y, et al. Catalytic transfer hydrogenation for stabilization of bio-oil oxygenates:Reduction of p-cresol and furfural over bimetallic Ni-Cu catalysts using isopropanol[J]. Fuel Processing Technology, 2015, 137:220-228.
doi: 10.1016/j.fuproc.2015.04.023 |
| 15 |
SHEN Zhibing, KE Ming, YU Pei, et al. Catalytic activities of Mo-modified Ni/Al2O3 catalysts for thioetherification of mercaptans and di-olefins in fluid catalytic cracking naphtha[J]. Transition Metal Chemistry, 2012, 37(6):587-593.
doi: 10.1007/s11243-012-9625-0 |
| 16 | LI Jingfeng, CHAI Yongming, LIU Bin, et al. The catalytic performance of Ni2P/Al2O3 catalyst in comparison with Ni/Al2O3 catalyst in dehydrogenation of cyclohexane[J]. Applied Catalysis A:General, 2014, 469:434-441. |
| 17 |
SHE Tiantian, CHU Xiaoning, ZHANG Huiling, et al. Ni-Mg/γ-Al2O3 catalyst for 4-methoxyphenol hydrogenation:Effect of Mg modification for improving stability[J]. Journal of Nanoparticle Research, 2018, 20(9):224.
doi: 10.1007/s11051-018-4329-9 |
| 18 | 刘伟, 张景成, 陈永生, 等. 一种高活性镍基丙酮加氢催化剂及制备方法:中国,112337474B[P]. 2023-05-09. |
| 19 | 刘伟, 陈永生, 许岩, 等. 一种羟乙基乙二胺合成哌嗪的催化剂及其制备方法、应用:中国,114247449B[P]. 2023-08-22. |
| 20 | 刘伟, 许岩, 陈永生, 等. 碱土金属对铜/三氧化二铝酯加氢催化剂性能的影响[J]. 无机盐工业, 2023, 55(9):140-144. |
| LIU Wei, XU Yan, CHEN Yongsheng, et al. Effect of alkaline earth metals on performance of Cu/Al2O3 ester hydrogenation cat-alyst[J]. Inorganic Chemicals Industry, 2023, 55(9):140-144. | |
| 21 |
ZHUANG Jianguo, YAN Siyan, ZHANG Peng, et al. Regulating the states of Ni species by controlling the silanols of MCM-41 support to promote the hydrogenation of maleic anhydride[J]. Fuel, 2023, 335:127030.
doi: 10.1016/j.fuel.2022.127030 |
| 22 |
HUANG Guan, SUN Zhichao, LIU Yingya, et al. Efficient Ni2P/SiO2 catalysts with enhanced performance for the hydrogenation of 4,6-dimethyldibenzothiophene and phenanthrene[J]. Industrial & Engineering Chemistry Research, 2023, 62(29):11428-11438.
doi: 10.1021/acs.iecr.3c01523 |
| 23 |
张因, 郭健健, 王杰, 等. 以NiAl-NO3-LDH为前驱体制备Ni-Al2O3催化剂及其催化乙酰丙酸加氢性能[J]. 高等学校化学学报, 2019, 40(8):1686-1696.
doi: 10.7503/cjcu20190068 |
|
ZHANG Yin, GUO Jianjian, WANG Jie, et al. Preparation of Ni-Al2O3 catalysts derived from hydrotalcite and its catalytic performance for hydrogenation of levulinic acid[J]. Chemical Journal of Chinese Universities, 2019, 40(8):1686-1696.
doi: 10.7503/cjcu20190068 |
|
| 24 | TIAN Weiping, GUO Shaofei, SHI Li. Ni-loaded catalyst containing activated clay for maleic anhydride hydrogenation to succinic anhydride[J]. China Petroleum Processing & Petrochemical Technology, 2011, 13(4):35-40. |
| 25 |
CHU Shuang, LI Xiang, ZHOU Xuerong, et al. Preparation of Ni2P supported on Al2O3 and B2O3 mixed oxides by temperature-programmed reduction of phosphate precursors with low P/Ni ratios[J]. Topics in Catalysis, 2020, 63(15):1379-1387.
doi: 10.1007/s11244-020-01344-6 |
| 26 |
LI Xiang, FENG Jianpeng, GUO Jingyu, et al. Preparation of Ni2P/Al2O3 by temperature-programmed reduction of a phosphate precursor with a low P/Ni ratio[J]. Journal of Catalysis, 2016, 334:116-119.
doi: 10.1016/j.jcat.2015.11.007 |
| 27 |
ZHANG Zhena, TANG Mingxiao, CHEN Jixiang. Effects of P/Ni ratio and Ni content on performance of γ-Al2O3-supported nickel phosphides for deoxygenation of methyl laurate to hydrocarbo-ns[J]. Applied Surface Science, 2016, 360:353-364.
doi: 10.1016/j.apsusc.2015.10.182 |
| 28 |
LI Kelun, WANG Rijie, CHEN Jixiang. Hydrodeoxygenation of anisole over silica-supported Ni2P,MoP,and NiMoP catalys- ts[J]. Energy & Fuels, 2011, 25(3):854-863.
doi: 10.1021/ef101258j |
| 29 | CECILIA J A, JIMÉNEZ-MORALES I, INFANTES-MOLINA A, et al. Influence of the silica support on the activity of Ni and Ni2P based catalysts in the hydrodechlorination of chlorobenzene.Study of factors governing catalyst deactivation[J]. Journal of Molecular Catalysis A:Chemical, 2013, 368-369:78-87. |
| 30 |
LEE Y K, OYAMA S T. Bifunctional nature of a SiO2-supported Ni2P catalyst for hydrotreating:EXAFS and FTIR studies[J]. Journal of Catalysis, 2006, 239(2):376-389.
doi: 10.1016/j.jcat.2005.12.029 |
| 31 | LONG Huali, XU Yan, ZHANG Xiaoqing, et al. Ni-Co/Mg-Al catalyst derived from hydrotalcite-like compound prepared by plasma for dry reforming of methane[J]. Journal of Energy Chemistry, 2013, 22(5):733-739. |
| 32 |
SAWHILL S J, LAYMAN K A, VAN WYK D R, et al. Thiophene hydrodesulfurization over nickel phosphide catalysts:Effect of the precursor composition and support[J]. Journal of Catalysis, 2005, 231(2):300-313.
doi: 10.1016/j.jcat.2005.01.020 |
| [1] | WANG Chao, SONG Guoliang, XIAO Han. Industrial application of THFS-2 sulfurized reforming prehydrogenation catalysts [J]. Inorganic Chemicals Industry, 2024, 56(5): 94-100. |
| [2] | DI Lu, WANG Weiguo, CHEN Juexian, WU Chuanshu. Study on preparation of transition metal-supported Silicalite-1 zeolite catalyst and its catalytic performance for furfural hydrogenation [J]. Inorganic Chemicals Industry, 2024, 56(4): 125-132. |
| [3] | ZHAN Sijin, LIU Shike, LIU Fei, YAO Mengqin, CAO Jianxin. Study on preparation and catalytic performance of ZnO-CeO2 [J]. Inorganic Chemicals Industry, 2024, 56(3): 137-143. |
| [4] | REN Qixia, YANG Kun, LIU Fei, YAO Mengqin, CAO Jianxin. Effect of promoter on physicochemical properties and catalytic performance of ZnO/ZrO2 [J]. Inorganic Chemicals Industry, 2024, 56(3): 144-154. |
| [5] | YANG Kun, REN Qixia, DONG Yonggang, LIU Fei, YAO Mengqin, CAO Jianxin. Effect of calcination temperature on catalytic performance of ZnGaZrO x /SAPO-34 [J]. Inorganic Chemicals Industry, 2024, 56(2): 136-145. |
| [6] | WANG Yansu, FENG Qing, LIU Guozhu, YU Haibin, SUN Yanmin, NAN Jun. Advances in encapsulated catalysts for propane dehydrogenation [J]. Inorganic Chemicals Industry, 2024, 56(11): 95-104. |
| [7] | LIU Wei, XU Yan, CHEN Yongsheng, SUN Chunhui, ZHANG Jingcheng, ZHU Jinjian, LIU Yang. Effect of alkaline earth metals on performance of Cu/Al2O3 ester hydrogenation catalyst [J]. Inorganic Chemicals Industry, 2023, 55(9): 140-144. |
| [8] | MA Zhiyuan, LÜ Dawei, WANG Hui, JIN Nannan, ZHU Jinjian, ZHANG Jingcheng. Industrial application of THDS-2/3 catalyst in capacity expansion of hydrofining plant [J]. Inorganic Chemicals Industry, 2023, 55(8): 140-144. |
| [9] | WANG Yansu, LIU Guozhu, YU Haibin. Research progress of platinum-based propane dehydrogenation catalysts [J]. Inorganic Chemicals Industry, 2023, 55(7): 1-9. |
| [10] | JIA Yuhong, HU Zhongpan, WANG Kunyuan, HAN Jingfeng, WEI Yingxu, LIU Zhongmin. Co anchored on silanol nests of S-1 zeolite for propane dehydrogenation to propylene [J]. Inorganic Chemicals Industry, 2023, 55(5): 121-127. |
| [11] | LI Haitao,ZHAO Yinfeng. Nitriding SBA-15 loaded highly dispersed transition metals for efficient direct dehydrogenation of propane [J]. Inorganic Chemicals Industry, 2023, 55(2): 141-148. |
| [12] | WANG Yansu, LIU Guozhu, YU Haibin. Research progress of non-precious metal catalysts for propane dehydrogenation [J]. Inorganic Chemicals Industry, 2023, 55(12): 1-11. |
| [13] | LIU Zenghui, SONG Bingjie, CHEN He, SUN Peiyong, CHAI Wenzheng, ZHANG Shenghong, FU Songbao, YAO Zhilong. Effect of structure of copper-based catalyst on adsorption and diffusion of mixed solution of unsaturated aldehydes and fatty alcohol [J]. Inorganic Chemicals Industry, 2023, 55(12): 152-158. |
| [14] | WANG Peng, ZHAO Shanshan, LU Yanfei, LI Shisong, WANG Chunlei, SHU Chang, ZHANG Dongsheng. Study on preparation of copper-based materials and its catalytic performance for hydrogenation of dimethyl succinate [J]. Inorganic Chemicals Industry, 2023, 55(10): 145-152. |
| [15] | QIU Xiaokui, ZHANG Ruofan, WANG Xiaoyan, WANG Hailong, ZHANG Qixue, WAN Chao, XU Lixin. Research on bamboo shavings carbon supported ruthenium catalysts for hydrogen generation from photocatalytic hydrolysis of ammonia borane [J]. Inorganic Chemicals Industry, 2023, 55(10): 153-158. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||