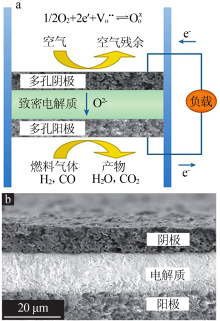
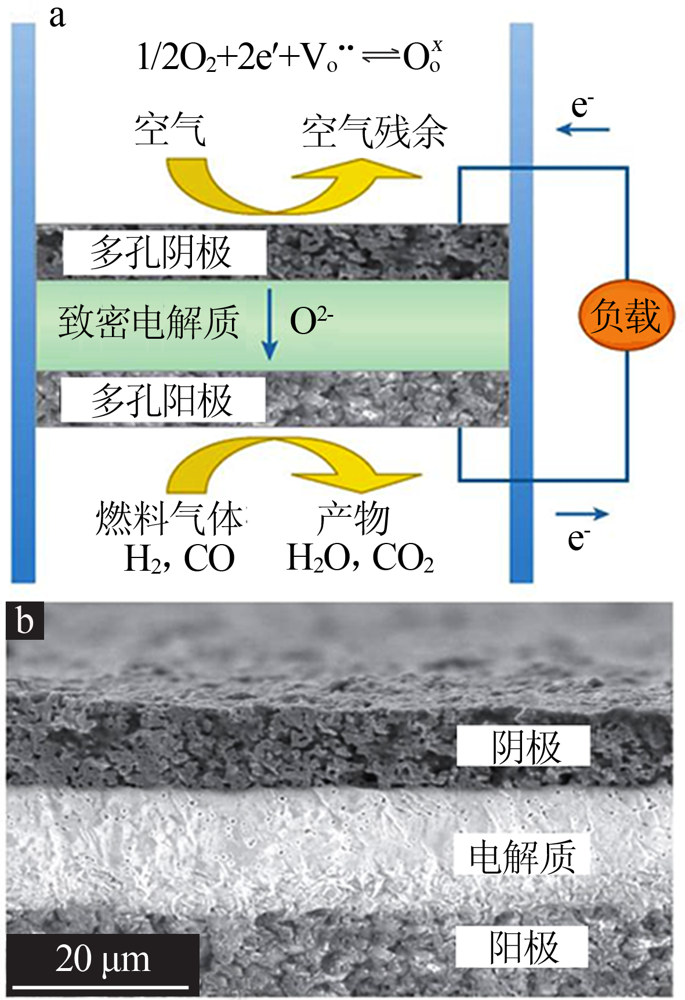
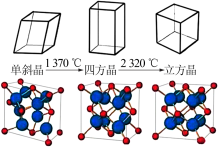
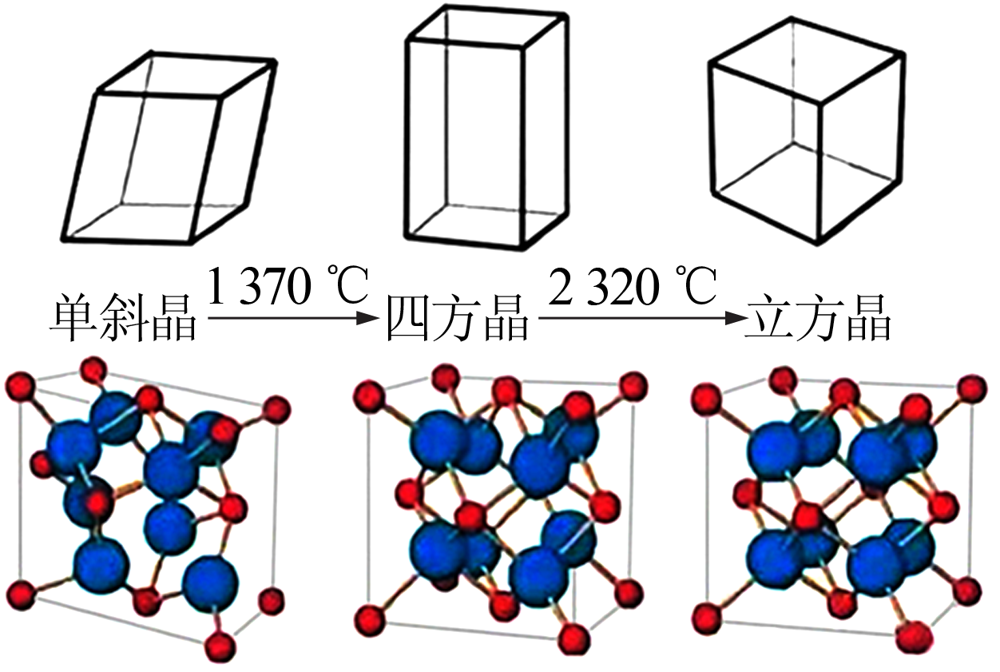
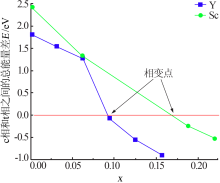
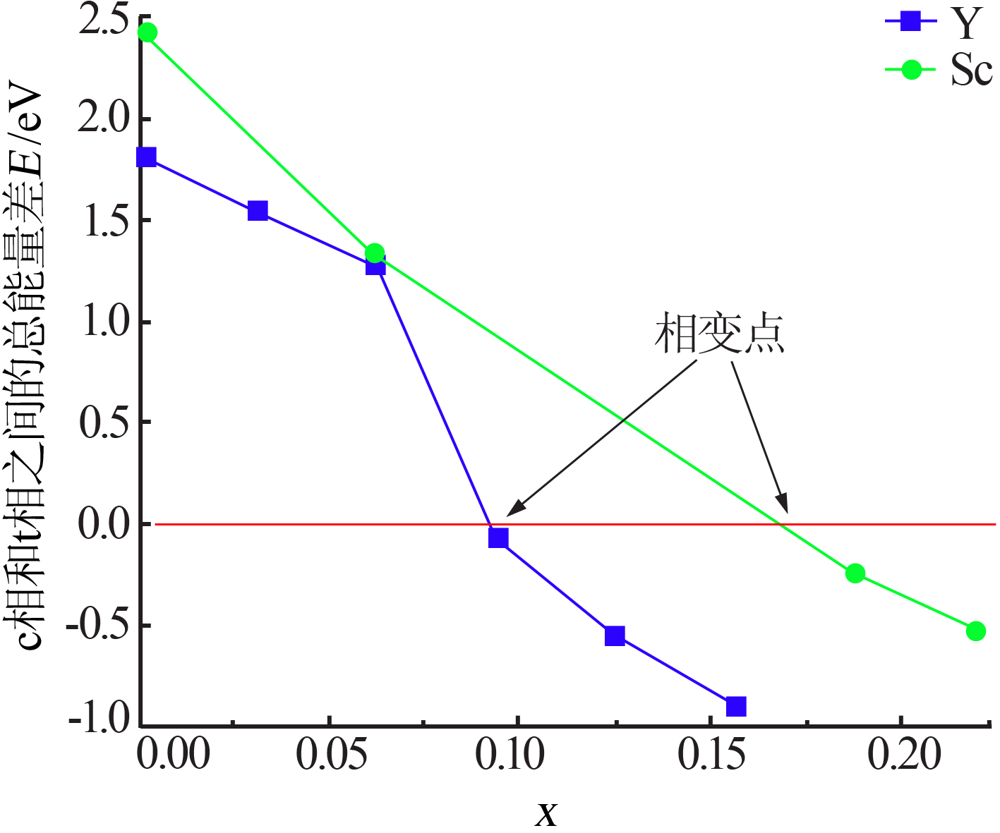
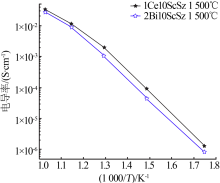
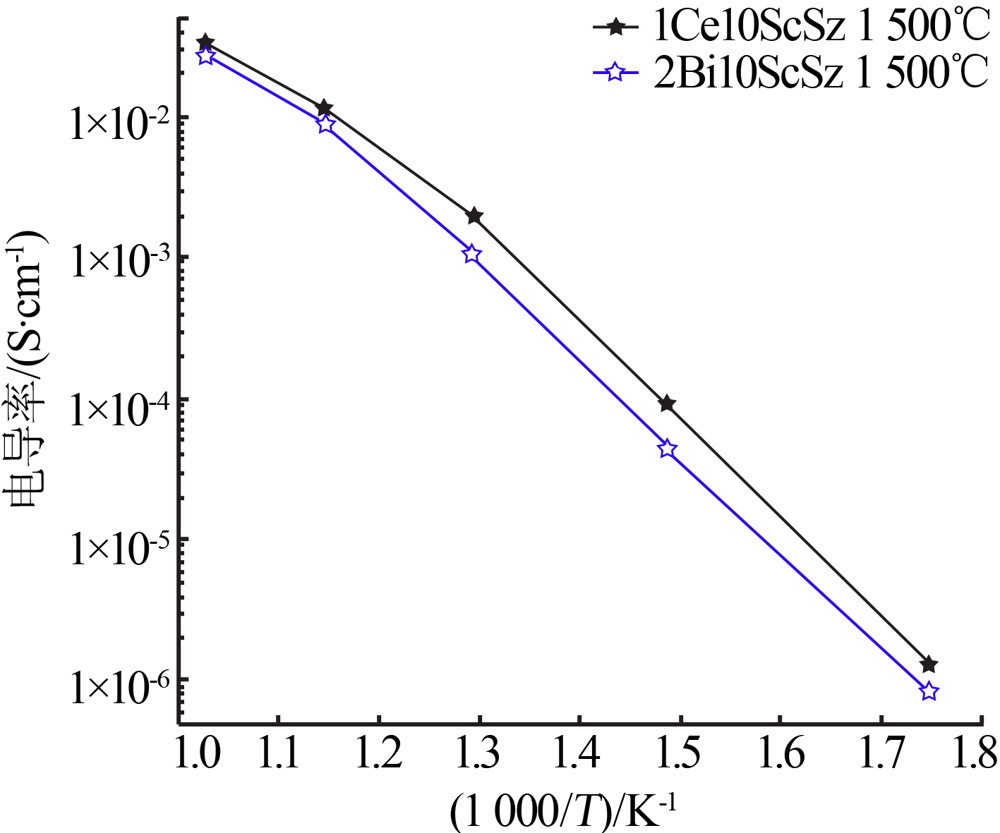
Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (6): 87-94.doi: 10.19964/j.issn.1006-4990.2020-0369
• Reviews and Special Topics • Previous Articles Next Articles
Research progress of conductivity of nano ScSZ-based electrolyte at medium and low temperature
Liang Tianquan1,2,3( ),Guo Yan1,Chen Xiyong1,2,Zhan Feng1,2,Zhang Xiuhai1,2,Zeng Jianmin1,2,3
),Guo Yan1,Chen Xiyong1,2,Zhan Feng1,2,Zhang Xiuhai1,2,Zeng Jianmin1,2,3
- 1. School of Resources,Environment and Materials,Guangxi University,Nanning 530004,China
2. Guangxi Key Laboratory of Processing for Non-ferrous Metals and Featured Materials
3. Center of Ecological Collaborative Innovation for Aluminum Industry in Guangxi
-
Received:2020-06-26Online:2021-06-10Published:2021-07-08
CLC Number:
Cite this article
Liang Tianquan,Guo Yan,Chen Xiyong,Zhan Feng,Zhang Xiuhai,Zeng Jianmin. Research progress of conductivity of nano ScSZ-based electrolyte at medium and low temperature[J]. Inorganic Chemicals Industry, 2021, 53(6): 87-94.
share this article
Table 1
Preparation method and conductivity of CeScSZ"
| 电解质 | 制备方法 | 测试 温度/℃ | 电导率/ (S·cm-1) |
|---|---|---|---|
| 1Ce10ScSZ[ | 固相合成:1 400 ℃烧结 | 600 | 0.046(高纯度) 0.033(低纯度) |
| 1Ce10ScSZ[ | 1 500 ℃烧结2 h,平均粒 径为5.9 μm | 800 | 0.140 |
| 1Ce10ScSZ[ | 甘氨酸-硝酸盐法(MW- GNP):微波中固溶燃烧合成,700 ℃煅烧2 h,1 300 ℃ 烧结5 h | 500~ 800 | 0.033~ 0.184 |
| 1Ce9ScSZ[ | 固相合成:1 335 ℃煅烧 6 h,1 550 ℃烧结10 h | 700 600 | 0.032 0.018 8 |
| 1Ce10ScSZ[ | 水热法:800 ℃煅烧2 h, 1 400 ℃烧结2 h,平均粒 径为20.2 nm | 800 | 0.131 |
| 1Ce10ScSZ[ | 微波烧结法:在微波炉中1 335 ℃烧结15 min | 800 | 0.280 |
| 1Ce10ScSZ[ | 新型固液方法(SLM): 1 000 ℃煅烧2 h,1 550 ℃煅烧5 h | 800 | 0.140 |
| 1Ce10ScSZ[ | 化学共沉淀法:1 500 ℃烧结5 h | 800 | 0.040 |
| 1Ce10ScSZ[ | 溶胶-凝胶法:600 ℃煅烧24 h,1 500 ℃烧结5 h | 600 | 0.011 4 |
| 1Ce10ScSZ[ | 沉淀-共沸蒸馏法:1 600 ℃ 烧结 | 800 | 0.084 |
Table 2
Conductivity of other cation doped ScSZ electrolyte"
| 电解质 | 制备方法 | 测试 温度/℃ | 电导率/ (S·cm-1) |
|---|---|---|---|
| 4Y4ScSZ[ | 溶胶凝胶-滴涂沉积法:1 000 ℃煅烧6 h,平均直径为144 nm,活化能为0.73 eV | 700 | 0.120 |
| 1Eu10ScSZ[ | 固相合成法:降低β→c转变温度,室温下为立方相 | 500 | 0.002 |
| 3Mg9ScSZ[ | 尿素共沉淀合成法:1 200 ℃长时间处理不发生相变, 1 600 ℃ 3 h不影响致密性,相对密度约为97% | 700 800 | 0.028 0.073 |
| 1Nb10ScSZ[ | 固相合成法:1 300 ℃煅烧6 h,1 550 ℃烧结10 h,在650 ℃时比11ScSZ高80% | 650 700 | 0.018 8 0.031 8 |
| 3LaScSZ[ | 化学共沉淀法:800 ℃煅烧5 h, 1 550 ℃烧结6 h,室温下稳定立方相,晶粒为20~30 nm,晶界阻抗降低 | 500~ 660 | 0.021~ 0.024 |
| 1Mn10ScSZ[ | USP(超声喷雾热解)法:1 400 ℃煅烧10 h | 800 | 0.112 |
| 1Hf10ScSZ[ | 固相合成法:1 550 ℃烧结5 h | 600 | 0.008 8 |
| [1] | 吴雨泽, 王宇旸, 范红途. 固体氧化物燃料电池(SOFC)系统的研究现状[J]. 能源研究与利用, 2019(1):40-46. |
| [2] |
Ivers-Tiffee E, Weber A, Herbstritt D. Materials and technologies for SOFC-components[J]. Journal of the European Ceramic Society, 2001,21(10):1805-1811.
doi: 10.1016/S0955-2219(01)00120-0 |
| [3] |
Raharjo J, Ali S A M, Arjasa O P, et al. Synjournal and characteriza-tion of uniform-sized cubic ytterbium scandium co-doped zirconium oxide(1Yb10ScSZ) nanoparticles by using basic amino acid as or-ganic precursor[J]. International Journal of Hydrogen Energy, 2017,42(14):9274-9283.
doi: 10.1016/j.ijhydene.2016.06.230 |
| [4] |
Mohammadreza D, Muhamad A M Y, Noordin M Y. Investigation of three steps of hot corrosion process in Y2O3 stabilized ZrO2 coatings including nano zones[J]. Rare Earths, 2014,32(10):989-1002.
doi: 10.1016/S1002-0721(14)60173-3 |
| [5] |
Lu X, Zhao J, Wang X. Bi2O3 nanoporous filmfabricated by anodic oxidation and its photoelectrochemical performance[J]. Solid State Electrochem, 2013,17(4):1215-1219.
doi: 10.1007/s10008-012-1996-9 |
| [6] |
Patakangas J, Ma Y, Jing Y. Review and analysis of characterization methods and ionic conductivities for lowtemperature solid oxide fuel cells(LT-SOFC)[J]. Power Sources, 2014,263:315-331.
doi: 10.1016/j.jpowsour.2014.04.008 |
| [7] |
Rajeswari K, Suresh M B, Chakravarty D, et al. Effect of nano-grain size on the ionic conductivity of spark plasma sintered 8YSZ elec-trolyte[J]. International Journal of Hydrogen Energy, 2012,37(1):511-517.
doi: 10.1016/j.ijhydene.2011.09.018 |
| [8] | Jiang H, Guo R, Ren J. Effects of ZnO additive on sinterability and electrochemical performances of 8YSZ electrolyte[J]. Journal of the Chinese Ceramic society, 2010,33(8):1434-1439. |
| [9] | Tiunova O V, Khabas T A, Bredikhin S I, et al. Zirconia-based solid electrolyte obtained by tape casting[J]. Inorganic Materials:App-lied Research, 2016,7(2):278-284. |
| [10] |
Souza J P, Grosso R L, Muccillo R, et al. Phase composition and io-nic conductivity of zirconia stabilized with scandia and europia[J]. Materials Letters, 2018,229:53-56.
doi: 10.1016/j.matlet.2018.06.091 |
| [11] |
Ng C K, Ramesh S, Tan C Y, et al. Microwave sintering of ceria-do-ped scandia stabilized zirconia as electrolyte for solid oxide fuel cell[J]. International Journal of Hydrogen Energy, 2016,41(32):14184-14190.
doi: 10.1016/j.ijhydene.2016.06.146 |
| [12] |
Kumar A, Jaiswal A, Sanbui M, et al. Scandia stabilized zirconia-ceria solid electrolyte (xSc1CeSZ,5<x<11) for IT-SOFCs:Struc-ture and conductivity studies[J]. Scripta Materialia, 2016,121:10-13.
doi: 10.1016/j.scriptamat.2016.04.023 |
| [13] |
Fabbri E, Pergolesi D, Traversa E. Electrode materials:A challenge for the exploitation of protonic solid oxide fuel cells[J]. Science and Technology of Advanced Materials, 2010,11(4):044301-044309.
doi: 10.1088/1468-6996/11/4/044301 |
| [14] | Lakshmi V V, Bauri R, Gandhi A S, et al. Synjournal and characte-rization of nanocrystalline ScSZ electrolyte for SOFCs[J]. Interna-tional Journal of Hydrogen Energy, 2011,36(22):14936-14942. |
| [15] | Xue Q, Huang X, Wang L, et al. Computational and experimental investigations of defect interaction and ionic conductivity in doped zirconia[J]. Physical Review Applied, 2018,10(1).Doi: 10.1103/PhysRevApplied.10.014032. |
| [16] |
Tan J, Su Y, Tang H, et al. Effect of calcined parameters on micro-structure and electrical conductivity of 10Sc1CeSZ[J]. Journal of Alloys and Compounds, 2016,686:394-398.
doi: 10.1016/j.jallcom.2016.06.039 |
| [17] |
Chen X, Zhou M, Shi J, et al. Microstructure and electrical conduc-tivity of A-site fully stoichiometric Na0.5+xBi0.5-xTiO3-δ with different Na/Bi ratios[J]. Ceramics International, 2019,45(9):11438-11447.
doi: 10.1016/j.ceramint.2019.03.011 |
| [18] | 徐高峰. 氧化锆纳米粉体的制备及稳定性研究[D]. 郑州:郑州大学, 2017. |
| [19] | Raza M, Cornil D, Cornil J, et al. Oxygen vacancy stabilized zirco-nia (OVSZ):a joint experimental and theoretical study[J]. Scri-pta Materialia, 2016,124:26-29. |
| [20] | Chen C, Liang T, Guo Y, et al. Effect of scandia content on the hot corrosion behavior of Sc2O3 and Y2O3 co-doped ZrO2 in Na2SO4+ V2O5 molten salts at 1 000 ℃[J]. Corrosion Science, 2019,158. Doi: 10.1016/j.corsci.2019.108094. |
| [21] |
Borik M A, Bredikhin S I, Kulebyakin A V, et al. Melt growth,st-ructure and properties of(ZrO2)1-x(Sc2O3) x solid solution crystals (x=0.035-0.11)[J]. Journal of Crystal Growth, 2016,443:54-61.
doi: 10.1016/j.jcrysgro.2016.03.004 |
| [22] | Gao Z, MognI L V, Miller E C, et al. A perspective on low-tempera-ture solid oxide fuel cells[J]. Energy & Environmental Science, 2016,9(5):1602-1644. |
| [23] | Brodnikovska I, Korsunska N, Khomenkova L, et al. Grains,grain boundaries and total ionic conductivity of 10Sc1CeSZ and 8YSZ solid electrolytes affected by crystalline structure and dopant co-ntent[J]. Materials Today:Proceedings, 2019(6):79-85. |
| [24] |
Fini D, Badwal S P S, Giddey S, et al. Evaluation of Sc2O3-CeO2- ZrO2 electrolyte-based tubular fuel cells using activated charcoal and hydrogen fuels[J]. Electrochimica Acta, 2018,259:143-150.
doi: 10.1016/j.electacta.2017.10.140 |
| [25] | Jais A A, Ali S A M, Anwar M, et al. Enhanced ionic conductivity of scandia-ceria-stabilized-zirconia(10Sc1CeSZ) electrolyte synt-hesized by the microwave-assisted glycine nitrate process[J]. Ce-ramics International, 2017,43(11):8119-8125. |
| [26] |
Tan J, Su Y, Tang H, et al. Effect of calcined parameters on micro-structure and electrical conductivity of 10Sc1CeSZ[J]. Journal of Alloys and Compounds, 2016,686:394-398.
doi: 10.1016/j.jallcom.2016.06.039 |
| [27] |
Liu M, He C R, Wang W G, et al. Synjournal and characterization of 10Sc1CeSZ powders prepared by a solid-liquid method for electro-lyte-supported solid oxide fuel cells[J]. Ceramics International, 2014,40(4):5441-5446.
doi: 10.1016/j.ceramint.2013.10.129 |
| [28] |
Liu M, He C, Wang J, et al. Investigation of (CeO2)x(Sc2O3)(0.11-x)(ZrO2)0.89(x=0.01~0.10) electrolyte materi-als for intermediate-temperature solid oxide fuel cell[J]. Journal of Alloys and Compounds, 2010,502(2):319-323.
doi: 10.1016/j.jallcom.2009.12.134 |
| [29] |
Abbas H A, Argirusis C, Kilo M, et al. Preparation and conductivity of ternary scandia-stabilised zirconia[J]. Solid State Ionics, 2011,184(1):6-9.
doi: 10.1016/j.ssi.2010.10.012 |
| [30] |
Wang Z, Cheng M, Bi Z, et al. Structure and impedance of ZrO2 do-ped with Sc2O3 and CeO2[J]. Materials Letters, 2015,59(19/20):2579-2582.
doi: 10.1016/j.matlet.2004.07.065 |
| [31] | 刘丽伟. Bi2O3/YSZ和Bi2O3/YBSZ电解质的合成制备和表征[D]. 杭州:浙江大学, 2016. |
| [32] |
Bai B, Sammes N M, Smirnova A L. Physical and electrochemical characterization of Bi2O3-doped scandia stabilized zirconia[J]. Journal of Power Sources, 2008,176(1):76-81.
doi: 10.1016/j.jpowsour.2007.10.074 |
| [33] |
Hirano M, Oda T, Ukai K, et al. Suppression of rhombohedral-phase appearance and low-temperature sintering of scandia-doped cubic-zirconia[J]. Journal of the American Ceramic Society, 2002,85(5):1336-1338.
doi: 10.1111/j.1151-2916.2002.tb00275.x |
| [34] |
Hirano M, Oda T, Ukai K, et al. Effect of Bi2O3 additives in Sc sta-bilized zirconia electrolyte on a stability of crystal phase and elec-trolyte properties[J]. Solid State Ionics, 2003,158(3/4):215-223.
doi: 10.1016/S0167-2738(02)00912-8 |
| [35] |
Sarat S, Sammes N, Smirnova A. Bismuth oxide doped scandia-sta-bilized zirconia electrolyte for the intermediate temperature solid oxide fuel cells[J]. Journal of Power Sources, 2006,160(2):892-896.
doi: 10.1016/j.jpowsour.2006.02.007 |
| [36] |
Bai B, Mcphee W A, Smirnova A, et al. A comparison and charac-terization of CeO2-doped and Bi2O3-doped scandia stabilized zi-rconia as IT-SOFC electrolytes[J]. ECS Transactions, 2007,7(1):2213-2221.
doi: 10.1149/1.2729337 |
| [37] | Agarkov D A, Borik M A, Bredikhin S I, et al. Transport properties of single crystals of solid electrolytes based on ZrO2-Sc2O3 co-do-ped by scandia,yttria,ytterbia and ceria[J]. Chemical Probhems, 2019,2(17):235-245. |
| [38] |
Omar S, Najib W B, Chen W, et al. Electrical conductivity of 10 mol % Sc2O3-1 mol % M2O3-ZrO2 ceramics[J]. Journal of the American Ceramic Society, 2012,95(6):1965-1972.
doi: 10.1111/jace.2012.95.issue-6 |
| [39] |
Jeon H J, Kim K J, Kim M Y, et al. Fabrication and electrochemical characterization of SOFC single cell with 6Yb4ScSZ electrolyte po-wder by tape-casting and co-sintering[J]. Journal of the Ceramic Society of Japan, 2015,123(1436):229-234.
doi: 10.2109/jcersj2.123.229 |
| [40] |
Yuan F, Wang J, Miao H, et al. Investigation of the crystal structure and ionic conductivity in the ternary system(Yb2O3)x-(Sc2O3)(0.11-x)- (ZrO2)0.89(x=0~0.11)[J]. Journal of Alloys and Compounds, 2013,549:200-205.
doi: 10.1016/j.jallcom.2012.09.089 |
| [41] |
Alfeche D M, Cervera R B. Highly conducting Sc and Y co-doped ZrO2 thin film solid electrolyte on a porous Ni/YSZ electrode pre-pared via simple drop-coating method[J]. Ceramics International, 2020,46(8):10561-10567.
doi: 10.1016/j.ceramint.2020.01.058 |
| [42] | Accardo G, Agli G D, Frattini D, et al. Electrical behaviour and mi-crostructural characterization of magnesia co-doped ScSZ nanopo-wders synthesized by urea co-precipitation[J]. Chemical Engineer-ing Transactions, 2017,57:1345-1350. |
| [43] | Kumar A, Singh R P, Singh S, et al. Phase stability and ionic con-ductivity of cubic xNb2O5-(11-x)Sc2O3-ZrO2(0≤x≤4)[J]. Jour-nal of Alloys and Compounds, 2017,703:643-651. |
| [44] | Pastor M, Prasad A, Biswas K, et al. Microstructural and impedance study of nanocrystalline lanthana-doped scandia-stabilized zirco-nia[J]. Journal of Nanoparticle Research, 2012,14(8):1-11. |
| [45] | Choi Y, Lee S, Wackerl J, et al. Fabrication of scandia-stabilized zirconia electrolyte with a porous and dense composite layer for so-lid oxide fuel cells[J]. Ceramics International, 2012,38:5485-5488. |
| [46] |
Omar S, Najib W B, Bonanos N. Conductivity ageing studies on 1M10ScSZ(M 4+=Ce,Hf)[J]. Solid State Ionics, 2011,189(1):100-106.
doi: 10.1016/j.ssi.2011.02.016 |
| [47] |
Guo X. Roles of alumina in zirconia for functional applications[J]. Journal of the American Ceramic Society, 2003,86(11):1867-1873.
doi: 10.1111/jace.2003.86.issue-11 |
| [48] |
Lybye D, Liu Y L. A study of complex effects of alumina addition on conductivity of stabilised zirconia[J]. Journal of the European Ceramic Society, 2006,26(4/5):599-604.
doi: 10.1016/j.jeurceramsoc.2005.06.009 |
| [49] |
Guo C X, Wang J X, He C R. et al. Effect of alumina on the proper-ties of ceria and scandia co-doped zirconia for electrolyte-suppo-rted SOFC[J]. Ceramics International, 2013,39(8):9575-9582.
doi: 10.1016/j.ceramint.2013.05.076 |
| [50] |
Feighery A J, Irvine J T S. Effect of Alumina additions upon elec-trical properties of 8mol% yttria-stabilised zirconia[J]. Solid State Ionics, 1999,121(1/2/3/4):209-216.
doi: 10.1016/S0167-2738(99)00015-6 |
| [51] |
Lv Z, Guo R, Yao P, et al. Effects of Al2O3 and/or CaO on properties of yttria stabilized zirconia electrolyte doped with multi-elemen-ts[J]. Materials & Design, 2007,28(4):1399-1403.
doi: 10.1016/j.matdes.2005.12.004 |
| [52] |
Agarkov D A, Borik M A, Bredikhin S I, et al. Structure and trans-port properties of zirconia crystals co-doped by scandia,ceria and yttria[J]. Journal of Materiomics. 2019,5(2):273-279.
doi: 10.1016/j.jmat.2019.02.004 |
| [53] | Lv Z G, Yao P, Guo R S, et al. Study on zirconia solid electrolytes doped by complex additives[J]. Materials Science and Engineer-ing:A, 2007,458(1/2):355-360. |
| [54] |
Knoner G, Reimann K, Rower R, et al. Enhanced oxygen diffusivity in interfaces of nanocrystalline ZrO2·Y2O3[J]. Proceedings of the National Academy of Sciences, 2003,100(7):3870-3873.
doi: 10.1073/pnas.0730783100 |
| [55] |
Garcia-Barriocanal J, Rivera-Calzada A, Varela M, et al. Colossal ionic conductivity at interfaces of epitaxial ZrO2:Y2O3/SrTiO3 heterostructures[J]. Science, 2008,321(5889):676-680.
doi: 10.1126/science.1156393 |
| [56] | 程浩. 纳米氧化锆粉体抗团聚老化性的研究[D]. 武汉:湖北工业大学, 2009. |
| [57] |
Xue Q N, Huang X W, Zhang H, et al. Synjournal and characteriza-tion of high ionic conductivity ScSZ core/shell nanocomposites[J]. Journal of Rare Earths, 2017,35(6):567-573.
doi: 10.1016/S1002-0721(17)60949-9 |
| [58] |
Kazlauskas S, Kazakevicius E, Kezionis A. et al. Electrical proper-ties of scandia and ceria-stabilized zirconia ceramics[J]. Solid State Ionics, 2017,310:143-147.
doi: 10.1016/j.ssi.2017.08.008 |
| [59] |
Hongmin A O, Xiangsheng L I U, Zhang H, et al. Preparation of ac-andia stabilized zirconia powder using microwave-hydrothermal method[J]. Journal of Rare Earths, 2015,33(7):746-751.
doi: 10.1016/S1002-0721(14)60480-4 |
| [60] | Yao L, Liu W, Ou G, et al. Phase stability and high conductivity of ScSZ nanofibers:Effect of the crystallite size[J]. Journal of Mate-rials Chemistry A, 2015,3(20):10795-10800. |
| [61] |
Cho G Y, Lee Y H, Hong S W, et al. High-performance thin film solid oxide fuel cells with scandia-stabilized zirconia(ScSZ) thin film electrolyte[J]. International Journal of Hydrogen Energy, 2015,40(45):15704-15708.
doi: 10.1016/j.ijhydene.2015.09.124 |
| [1] | REN Qixia, YANG Kun, LIU Fei, YAO Mengqin, CAO Jianxin. Effect of promoter on physicochemical properties and catalytic performance of ZnO/ZrO2 [J]. Inorganic Chemicals Industry, 2024, 56(3): 144-154. |
| [2] | DONG Mingzhe, LI Kexin, YE Xiushen, MA Zhen, LI Shengting, LI Quan, WU Zhijian. Study on electrochemical properties of magnesium chloride molten salt hydrates [J]. Inorganic Chemicals Industry, 2024, 56(2): 51-56. |
| [3] | WANG Chen, HE Wei, SUN Mengyuan. Research on preparation of nano-bismuth oxide-enhanced chloride/magnesium oxide composites and their thermophysical properties [J]. Inorganic Chemicals Industry, 2024, 56(12): 120-126. |
| [4] | XU Xijun, LIN Jianfeng, LUO Xiongwei, ZHAO Jingwei, HUO Yanping. Recent progress of NASICON-type Na1+x Zr2Si x P3-x O12 solid electrolyte for sodium metal batteries [J]. Inorganic Chemicals Industry, 2024, 56(11): 1-14. |
| [5] | QIN Ye, LIU Chang, HAN Song, WANG Shuo. Study on conductivity of electrolyte in aqueous Zn-Mn battery [J]. Inorganic Chemicals Industry, 2024, 56(11): 132-138. |
| [6] | DU Haigang, WANG Xingyong, SUN Peiyong, CHEN He, FU Songbao, YAO Zhilong. Study on condensation performance between methyl propionate and formaldehyde catalyzed by Cs/SiO2-ZrO2 [J]. Inorganic Chemicals Industry, 2024, 56(11): 165-174. |
| [7] | CHEN Qi, LIAO Dankui, ZHANG Qingnian, YAN Jinsheng, HUANG Yu, CHEN Xiaopeng, TONG Zhangfa. Fast and efficient determination of quicklime activity by conductivity method [J]. Inorganic Chemicals Industry, 2023, 55(9): 114-120. |
| [8] | KANG Le, JING Maoxiang, LI Donghong, HU Xinyu, JIA Chunyan. Study on preparation and electrochemical performance of lithium aluminate nanorods modified solid electrolyte [J]. Inorganic Chemicals Industry, 2023, 55(8): 65-70. |
| [9] | GUO Jianye, WANG Dong, SU Lijun, LI Wenjing. Effect of aerogel doping on thermal insulation performance of glass fiber felt [J]. Inorganic Chemicals Industry, 2023, 55(11): 53-57. |
| [10] | ZHOU Min. Preparation of PANI/TIO composites and their application in polyurethane coatings [J]. Inorganic Chemicals Industry, 2023, 55(1): 112-117. |
| [11] | CHEN Mingsheng,LIU Peng,KONG Dewen,LI Yuan,YU Ke,HUANG Yansen,WU Ningbo,CHANG Juanjuan. Effect of external admixtures on mechanical and thermal conductivity of phosphogypsum composite cementitious materials [J]. Inorganic Chemicals Industry, 2022, 54(9): 113-118. |
| [12] | ZHANG Yu,QI Xiaoyu,FENG Mengyao,SUN Nannan,ZHAO Cuilian. Study on permeability of mesoporous silica antireflective film regulated by conductivity [J]. Inorganic Chemicals Industry, 2022, 54(8): 80-84. |
| [13] | ZHANG Yue,WANG Min,LI Jinli,ZHAO Youjing,WANG Huaiyou. Study on thermophysical properties of solar salt composites doped with nanoparticles [J]. Inorganic Chemicals Industry, 2022, 54(5): 54-60. |
| [14] | DONG Peng,ZHOU Yingjie,HOU Minjie,YANG Dongrong,DAI Yongnian,LIANG Feng. Research progress on Na3V2(PO4)3 cathode materials for sodium ion batteries [J]. Inorganic Chemicals Industry, 2022, 54(5): 1-10. |
| [15] | YAN Chen,LIU Hantao. Research progress on two-dimensional nanosheet-based composite phase change materials for thermal energy storage [J]. Inorganic Chemicals Industry, 2022, 54(2): 30-37. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||