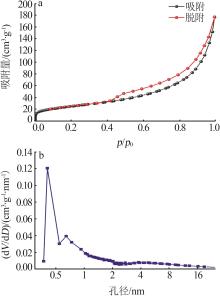
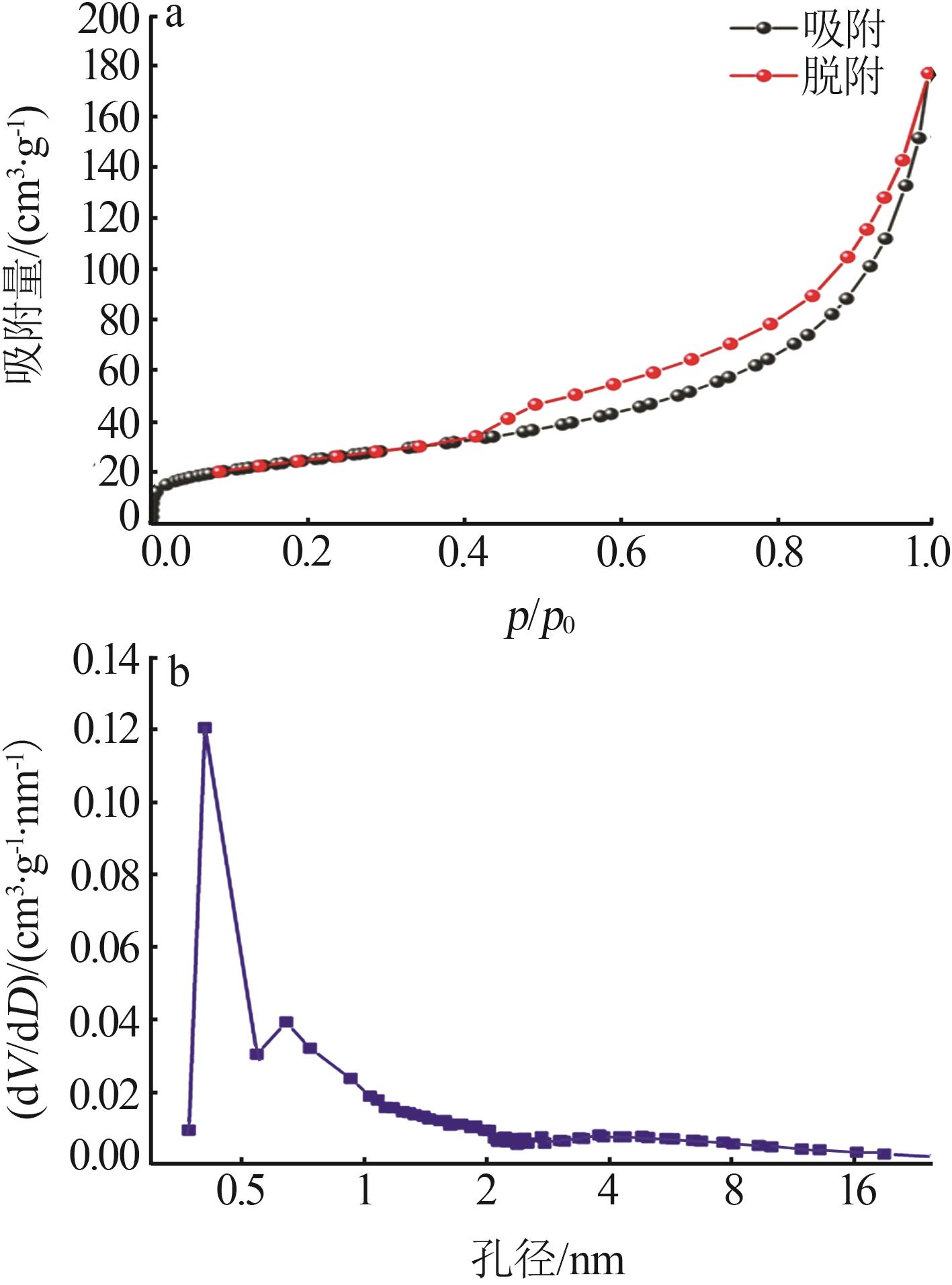
Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (12): 128-132.doi: 10.19964/j.issn.1006-4990.2023-0140
• Environment·Health·Safety • Previous Articles Next Articles
Synthesis and characterization of silico-manganese slag zeolite
DONG Xiongwei( ), HAN Fenglan(
), HAN Fenglan( ), HUA Wei, LI Maohui, AN Changcong, HUANG Yucai
), HUA Wei, LI Maohui, AN Changcong, HUANG Yucai
- School of Material Science and Engineering,North Minzu University,Yinchuan 750021,China
-
Received:2023-03-14Online:2023-12-10Published:2023-12-14 -
Contact:HAN Fenglan E-mail:1120329261@qq.com;625477897@qq.com
CLC Number:
Cite this article
DONG Xiongwei, HAN Fenglan, HUA Wei, LI Maohui, AN Changcong, HUANG Yucai. Synthesis and characterization of silico-manganese slag zeolite[J]. Inorganic Chemicals Industry, 2023, 55(12): 128-132.
share this article
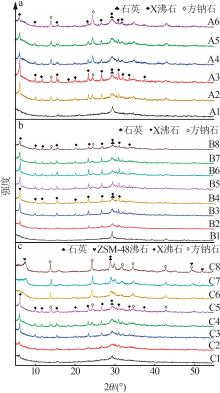
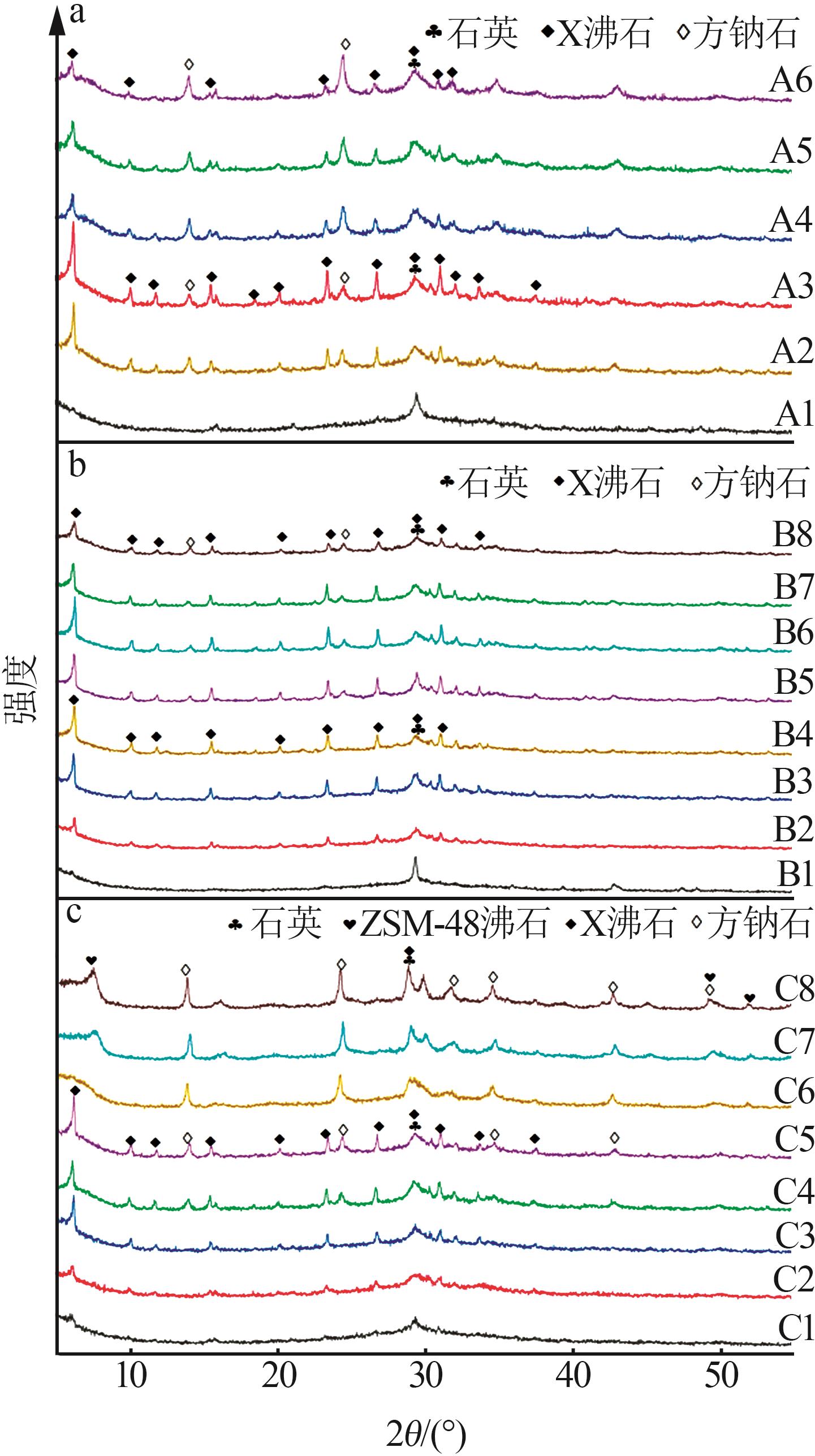
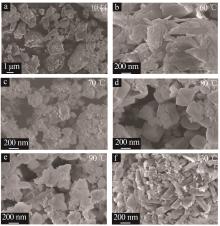
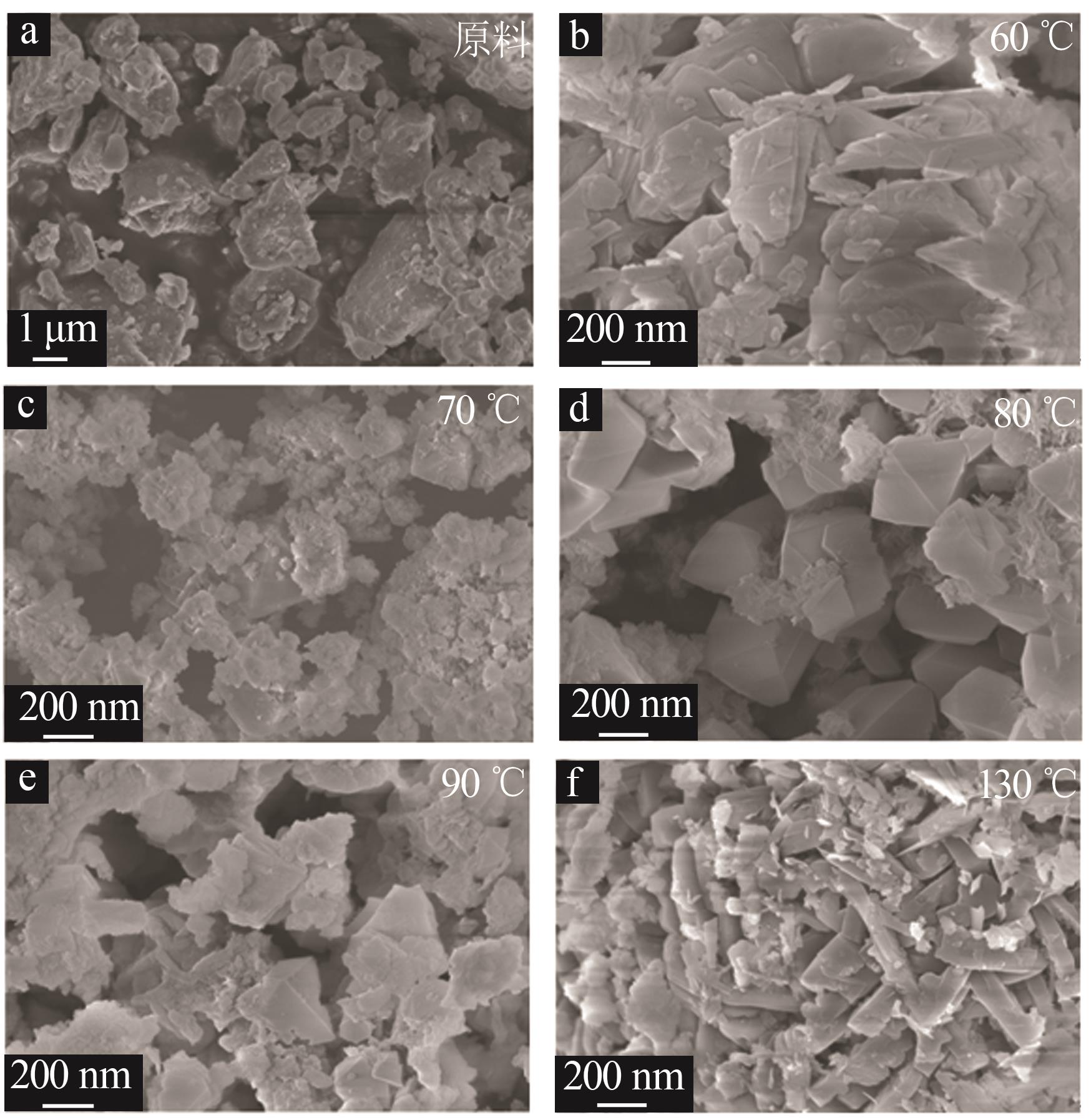
Table 1
Experimental ratio of X zeolite products"
样品 编号 | 氢氧化钠浓度/(mol·L-1) | 时间/ h | 温度/ ℃ | 样品 编号 | 氢氧化钠浓度/(mol·L-1) | 时间/ h | 温度/ ℃ |
|---|---|---|---|---|---|---|---|
| A1 | 0.2 | 10 | 90 | B6 | 0.5 | 10 | 90 |
| A2 | 0.4 | 10 | 90 | B7 | 0.5 | 11 | 90 |
| A3 | 0.5 | 10 | 90 | B8 | 0.5 | 12 | 90 |
| A4 | 0.8 | 10 | 90 | C1 | 0.5 | 8 | 60 |
| A5 | 1.0 | 10 | 90 | C2 | 0.5 | 8 | 70 |
| A6 | 1.5 | 10 | 90 | C3 | 0.5 | 8 | 80 |
| B1 | 0.5 | 3 | 90 | C4 | 0.5 | 8 | 90 |
| B2 | 0.5 | 6 | 90 | C5 | 0.5 | 8 | 100 |
| B3 | 0.5 | 7 | 90 | C6 | 0.5 | 8 | 110 |
| B4 | 0.5 | 8 | 90 | C7 | 0.5 | 8 | 120 |
| B5 | 0.5 | 9 | 90 | C8 | 0.5 | 8 | 130 |
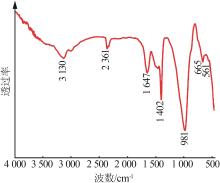
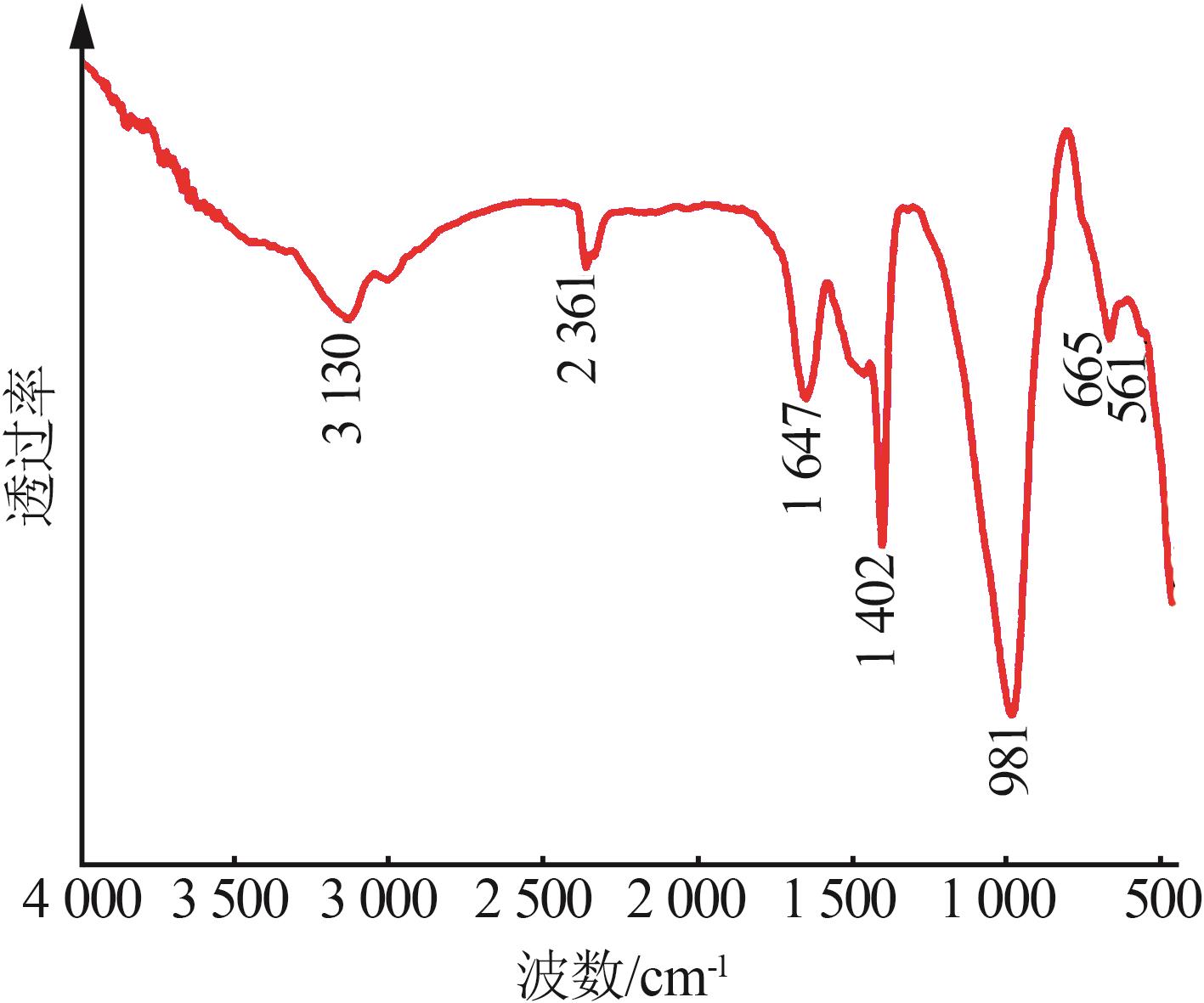
Table 2
Chemical compositions of silicon manganeseslag before and after treatment"
| 样品 | w(SiO2) | w (Al2O3) | w (Fe2O3) | w(CaO) | w(MgO) | w (K2O) | w (Na2O) | w(MnO) | w(其他) |
|---|---|---|---|---|---|---|---|---|---|
| 原渣 | 43.56 | 10.1 | 1.29 | 20.09 | 11.01 | 0.97 | 1.75 | 5.25 | 5.98 |
| 酸浸后 | 68.84 | 12.2 | 0.58 | 4.18 | 1.56 | 0.38 | 0.24 | 1.12 | 10.9 |
| 碱熔融 | 24.85 | 3.73 | 0.33 | 9.41 | 2.41 | 0.48 | 53.16 | 4.39 | 5.63 |
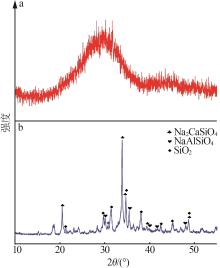
| 样品C3 | 46.55 | 13.11 | 0.47 | 16.99 | 7.37 | 0.16 | 10.19 | 3.89 | 1.27 |
| 1 | 刘强, 周飞, 张俊瑾, 等. 硅锰渣-固硫灰复合辅助性胶凝材料水化机理研究[J]. 硅酸盐通报, 2022, 41(5):1715-1723. |
| LIU Qiang, ZHOU Fei, ZHANG Junjin, et al. Hydration mechanism of silicomanganese slag-circulating fluidized bed combustion ash composite supplementary cementitious materials[J]. Bulletin of the Chinese Ceramic Society, 2022, 41(5):1715-1723. | |
| 2 | 周祥, 赵华堂, 李亮, 等. Si-Mn矿粉粒度对复合胶凝体系水化机理和力学性能的影响[J]. 材料导报, 2021, 35(S1):279-283. |
| ZHOU Xiang, ZHAO Huatang, LI Liang, et al. Effect of particle size of Si-Mn slag on hydraulic mechanism and mechanical property in composite cementitious system[J]. Materials Reports, 2021, 35(S1):279-283. | |
| 3 | 王晨晨, 王学志, 陈东林, 等. 基于正交试验的粉煤灰-硅锰渣再生混凝土力学性能研究[J]. 硅酸盐通报, 2022, 41(9):3190-3201. |
| WANG Chenchen, WANG Xuezhi, CHEN Donglin, et al. Mechanical properties of fly ash-silicon manganese slag recycled concrete based on orthogonal test[J]. Bulletin of the Chinese Ceramic Society, 2022, 41(9):3190-3201. | |
| 4 | 陈坤, 柯昌明, 张锦化. 硅锰渣基CaO-MgO-Al2O3-SiO2系矿渣微晶玻璃晶化性能研究[J]. 武汉科技大学学报, 2015, 38(5):346-350. |
| CHEN Kun, KE Changming, ZHANG Jinhua. Crystallization properties of silicomanganese slag-based CaO-MgO-Al2O3-SiO2 system glass-ceramics[J]. Journal of Wuhan University of Science and Technology, 2015, 38(5):346-350. | |
| 5 | 杨林, 张洪波, 曹建新. 硅锰渣制生态渗水砖[J]. 新型建筑材料, 2007, 34(6):27-30. |
| YANG Lin, ZHANG Hongbo, CAO Jianxin. Preparation of ecology water penetrable brick using siliconmanganese slag[J]. New Building Materials, 2007, 34(6):27-30. | |
| 6 | 辛鑫, 谭泽馨, 赵俊学, 等. 硅锰渣一步法制备微晶铸石的热处理工艺及性能[J]. 硅酸盐学报, 2022, 50(6):1677-1684. |
| XIN Xin, TAN Zexin, ZHAO Junxue, et al. Heat treatment process optimization of microcrystalline cast stone from silico-manganese slag by one-step preparation[J]. Journal of the Chinese Ceramic Society, 2022, 50(6):1677-1684. | |
| 7 | 吴迪秀, 罗柳, 贾玉娟, 等. 粉煤灰碱熔融-水热法合成A型沸石及吸附性能研究[J]. 硅酸盐通报, 2019, 38(6):1873-1877. |
| WU Dixiu, LUO Liu, JIA Yujuan, et al. Synthesis of A-zeolite from coal fly ash by alkali fusion-hydrothermal process and its adsorption research[J]. Bulletin of the Chinese Ceramic Society, 2019, 38(6):1873-1877. | |
| 8 | 崔家新, 王连勇, 卢思盟, 等. 几种不同产地粉煤灰水热法合成沸石性能探究[J]. 无机盐工业, 2022, 54(5):96-100. |
| CUI Jiaxin, WANG Lianyong, LU Simeng, et al. Research on performance of hydrothermally synthesized zeolite with fly ash from different producing areas[J]. Inorganic Chemicals Industry, 2022, 54(5):96-100. | |
| 9 | 金烈, 谭丽泉, 余梅, 等. 油页岩灰渣合成沸石及其对Cr(Ⅵ)的吸附研究[J]. 无机盐工业, 2018, 50(5):50-53. |
| JIN Lie, TAN Liquan, YU Mei, et al. Adsorption of Cr(Ⅵ) in aqueous solution by zeolite prepared from oil shale residue[J]. Inorganic Chemicals Industry, 2018, 50(5):50-53. | |
| 10 | 任英杰, 赵永红, 张广良. 煤矸石两步除铁合成低铁杂质4A沸石[J]. 无机盐工业, 2017, 49(1):42-45. |
| REN Yingjie, ZHAO Yonghong, ZHANG Guangliang. Synthesis of 4A zeolites with less Fe impurity from gangue after two removalir-on stages[J]. Inorganic Chemicals Industry, 2017, 49(1):42-45. | |
| 11 | 朱思雨, 李丽, 刘泽, 等. 硅锰渣复合粉煤灰水热合成NaA沸石及其表征[J]. 硅酸盐通报, 2022, 41(2):634-639. |
| ZHU Siyu, LI Li, LIU Ze, et al. Hydrothermal synthesis and characterization of zeolite NaA based on combination of silico-manganese slag and fly ash[J]. Bulletin of the Chinese Ceramic Society, 2022, 41(2):634-639. | |
| 12 | NATH S K, RANDHAWA N S, KUMAR S. A review on characteristics of silico-manganese slag and its utilization into construction materials[J]. Resources,Conservation and Recycling, 2022, 176:105946. |
| 13 |
TANAKA H, EGUCHI H, FUJIMOTO S, et al. Two-step process for synthesis of a single phase Na-A zeolite from coal fly ash by dialysis[J]. Fuel, 2006, 85(10/11):1329-1334.
doi: 10.1016/j.fuel.2005.12.022 |
| 14 |
LIU Liying, DU Tao, LI Gang, et al. Using one waste to tackle another:Preparation of a CO2 capture material zeolite X from laterite residue and bauxite[J]. Journal of Hazardous Materials, 2014, 278:551-558.
doi: 10.1016/j.jhazmat.2014.06.041 pmid: 25016453 |
| 15 |
REN Xiaoyu, LIU Shaojun, QU Ruiyang, et al. Synthesis and characterization of single-phase submicron zeolite Y from coal fly ash and its potential application for acetone adsorption[J]. Microporous and Mesoporous Materials, 2020, 295:109940.
doi: 10.1016/j.micromeso.2019.109940 |
| 16 |
CHEN Dan, HU Xin, SHI Lu, et al. Synthesis and characterization of zeolite X from lithium slag[J]. Applied Clay Science, 2012, 59-60:148-151.
doi: 10.1016/j.clay.2012.02.017 |
| 17 | LU X, LIU L, LIU H, et al. Zeolite-X synthesized from halloysite nanotubes and its application in CO2 capture[J]. Journal of the Taiwan Institute of Chemical Engineers, 2022,133. Doi:10.1016/j.jtice.2022.104281 . |
| [1] | DI Lu, WANG Weiguo, CHEN Juexian, WU Chuanshu. Study on preparation of transition metal-supported Silicalite-1 zeolite catalyst and its catalytic performance for furfural hydrogenation [J]. Inorganic Chemicals Industry, 2024, 56(4): 125-132. |
| [2] | HUANG Tao, HUANG Zili, XIAO Shuo, ZHENG Jiemiao, LIU Xiaofeng, WU Jilong. Experimental study on preparation of polyferric chloride from iron tailings acid leaching solution [J]. Inorganic Chemicals Industry, 2024, 56(2): 121-126. |
| [3] | XIA Guiying, YANG Liuchun, YUAN Zhiye. Study on direct leaching of rare earth elements from phosphogypsum with sulfuric acid [J]. Inorganic Chemicals Industry, 2024, 56(1): 107-113. |
| [4] | LI Chao, WANG Liping, DAI Yin, GAO Guimei, ZHANG Yunfeng, HONG Yu, XU Lijun, CUI Yongjie. Study on alkali fusion hydrothermal synthesis of 13X zeolite from high silicon tailings and its adsorption on lead,copper and zinc ions [J]. Inorganic Chemicals Industry, 2023, 55(9): 88-93. |
| [5] | HONG Meihua, GUO Zifeng, LIU Guanfeng, ZANG Jiazhong, YANG Keyu, YU Yonghua, ZHANG Dazhi, HUANG Shengjun. Progress and challenges of alkaline treatment for synthesis of hierarchical zeolites [J]. Inorganic Chemicals Industry, 2023, 55(6): 36-42. |
| [6] | ZHAO Hongjuan, WANG Jiujiang, LIU Yuhang, LI Ning, CAO Gengzhen, LIU Honghai. Impact of heterogenous crystal seeds on synthesis of NaY zeolite [J]. Inorganic Chemicals Industry, 2023, 55(4): 120-124. |
| [7] | YIN Xiaoyan,LIU Ning,SONG Feng,LONG Shuqi. Research on synthesis of CHA zeolite with different templates [J]. Inorganic Chemicals Industry, 2023, 55(2): 55-61. |
| [8] | TENG Jiayang, FENG Qingge, ZHANG Xuan, QIN Fanghong, FENG Jinghang, HU Jiawen, CHEN Chaohong. Study on preparation of pseudo-boehmite from aluminum dross resource treatment [J]. Inorganic Chemicals Industry, 2023, 55(11): 130-138. |
| [9] | LI Sen, WANG Jin, WANG Yingying, CAI Mingfang. Synthesis and modification of NaA zeolite and its application in polyurethane system [J]. Inorganic Chemicals Industry, 2023, 55(10): 78-85. |
| [10] | CUI Jiaxin,WANG Lianyong,LU Simeng,SUN Yanwen,WANG Rui,HE Yan,HAN Jianli. Research on performance of hydrothermally synthesized zeolite with fly ash from different producing areas [J]. Inorganic Chemicals Industry, 2022, 54(5): 96-100. |
| [11] | CUI Jiaxin,WANG Lianyong,LI Yao,HE Yan,WANG Rui,HAN Jianli. Preparation and properties characterization of water quenching slag-fly ash based 4A zeolite [J]. Inorganic Chemicals Industry, 2022, 54(4): 135-140. |
| [12] | XUE Haiyue,WANG Lianyong,LIU Xiangyu,HAN Jianli,YANG Yifan. Analysis and prospect of using fly ash based zeolite for infrared suppression of tail flame of aircraft [J]. Inorganic Chemicals Industry, 2022, 54(3): 23-30. |
| [13] | HONG Meihua,ZANG Jiazhong,WANG Lingtao,LIU Guanfeng,GONG Yupeng,PENG Xiaowei. Study on synthesis,modification and olefin removal performance of boron-doped Y zeolite [J]. Inorganic Chemicals Industry, 2022, 54(12): 139-147. |
| [14] | ZHU Yuanlu,GAO Ming,YAN Jiangyi,WANG Beifu. Research progress on volatile organic compounds(VOCs) adsorption by zeolites [J]. Inorganic Chemicals Industry, 2022, 54(11): 32-38. |
| [15] | PEI Yinchang,MO Shengpeng,XIE Qinglin,CHEN Nanchun. Study on mechanism of zeolite A synthesized from stellerite zeolite by two-step hydrothermal method [J]. Inorganic Chemicals Industry, 2022, 54(11): 59-64. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||