Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (1): 47-52.doi: 10.19964/j.issn.1006-4990.2023-0074
• Research & Development • Previous Articles Next Articles
Study on phase equilibria of aqueous ternary system of Na+(Mg2+),Ca2+∥Cl--H2O at 278.2 K
FENG Xia1( ), YU Xuefeng2, YAO Zhihao1, LUO Jun1,2, REN Siying1,2, ZHAO Zhixing1, YU Xudong1(
), YU Xuefeng2, YAO Zhihao1, LUO Jun1,2, REN Siying1,2, ZHAO Zhixing1, YU Xudong1( )
)
- 1. College of Materials and Chemistry & Chemical Engineering,Chengdu University of Technology,Chengdu 610059,China
2. Qinghai Salt Lake Industry Co.,Ltd.,Golmud 816099,China
-
Received:2023-02-15Online:2024-01-10Published:2024-01-18 -
Contact:YU Xudong E-mail:fengxiac413@126.com;xwdlyxd@126.com
CLC Number:
Cite this article
FENG Xia, YU Xuefeng, YAO Zhihao, LUO Jun, REN Siying, ZHAO Zhixing, YU Xudong. Study on phase equilibria of aqueous ternary system of Na+(Mg2+),Ca2+∥Cl--H2O at 278.2 K[J]. Inorganic Chemicals Industry, 2024, 56(1): 47-52.
share this article
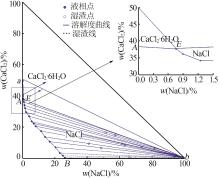
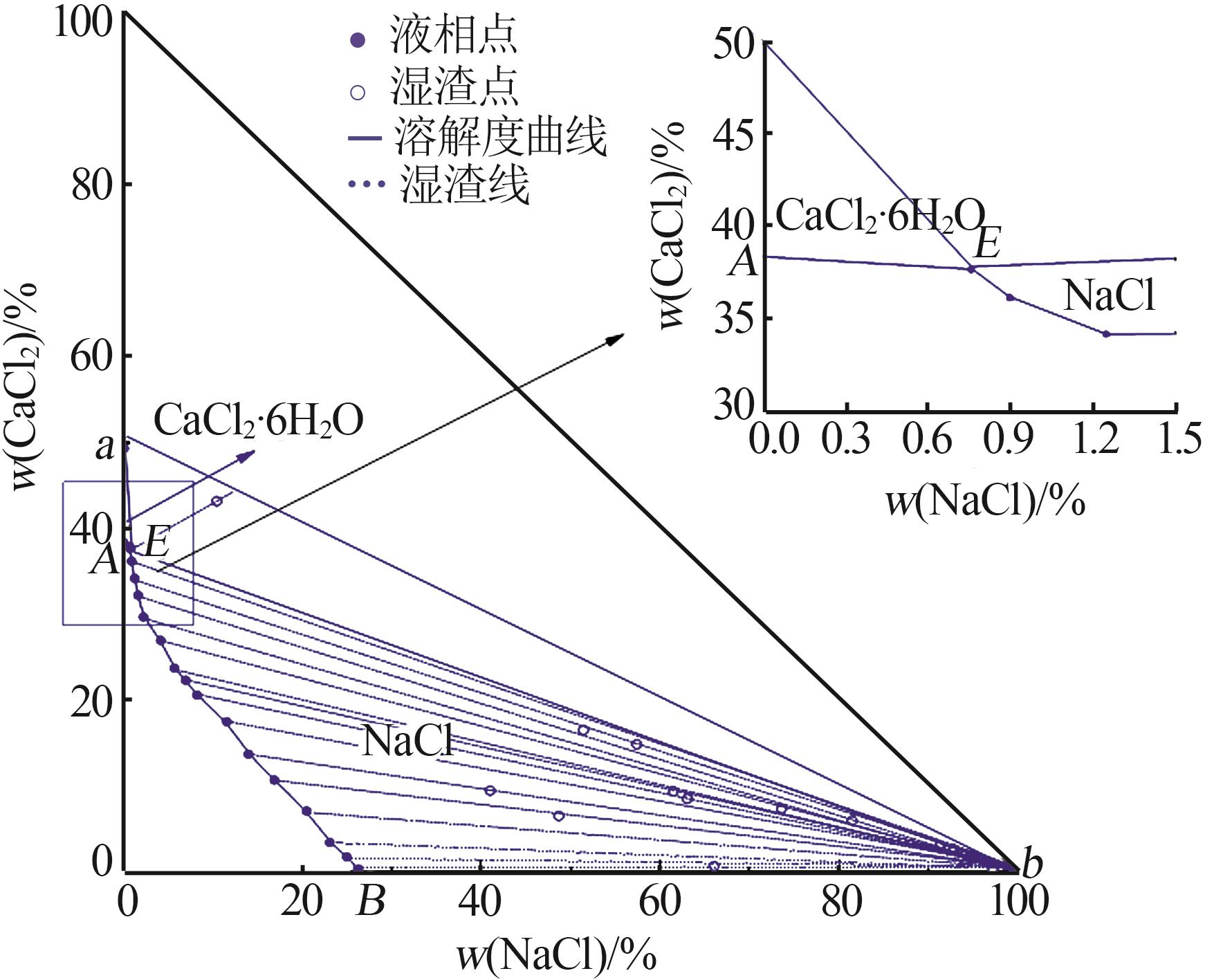
Table 1
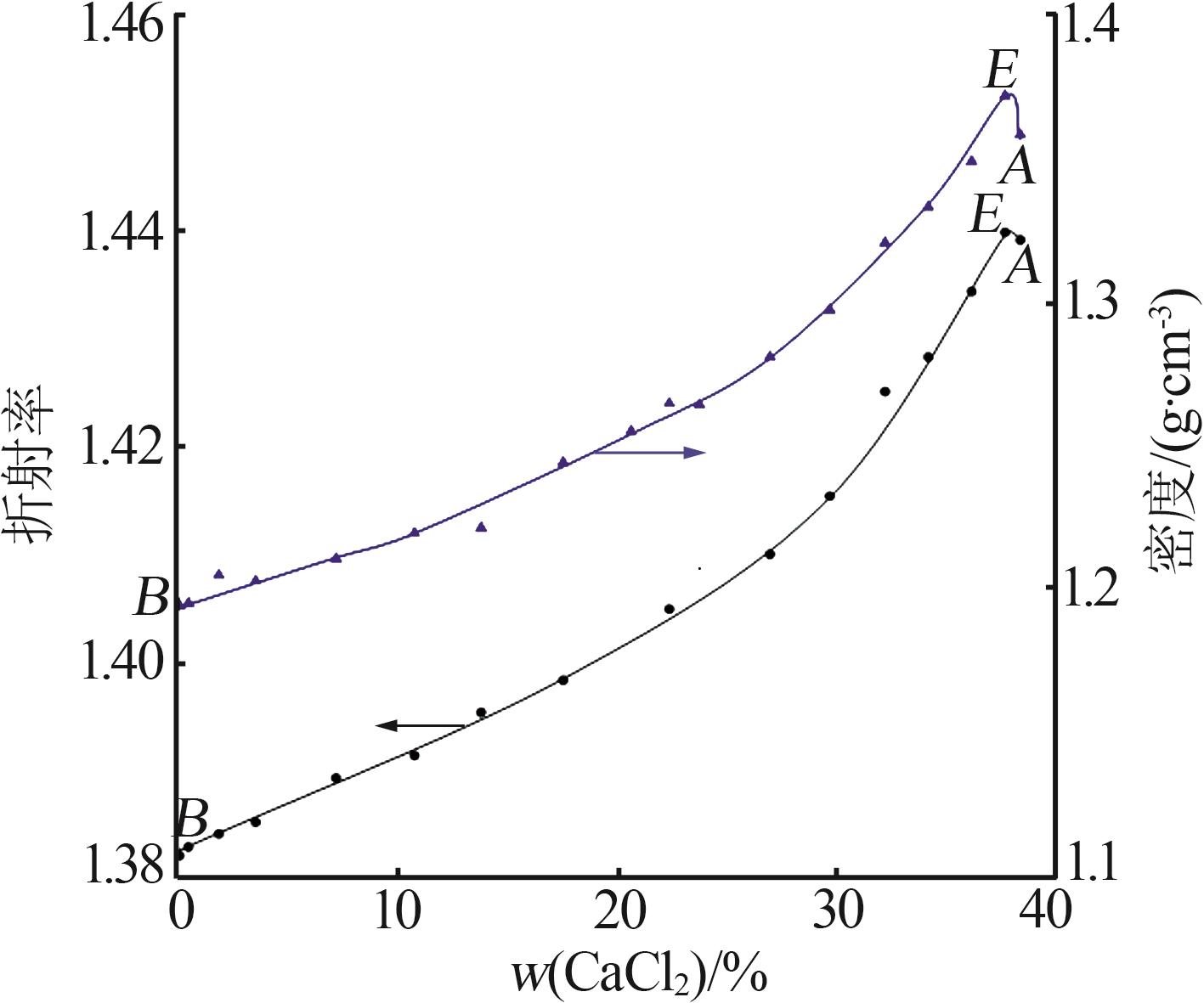
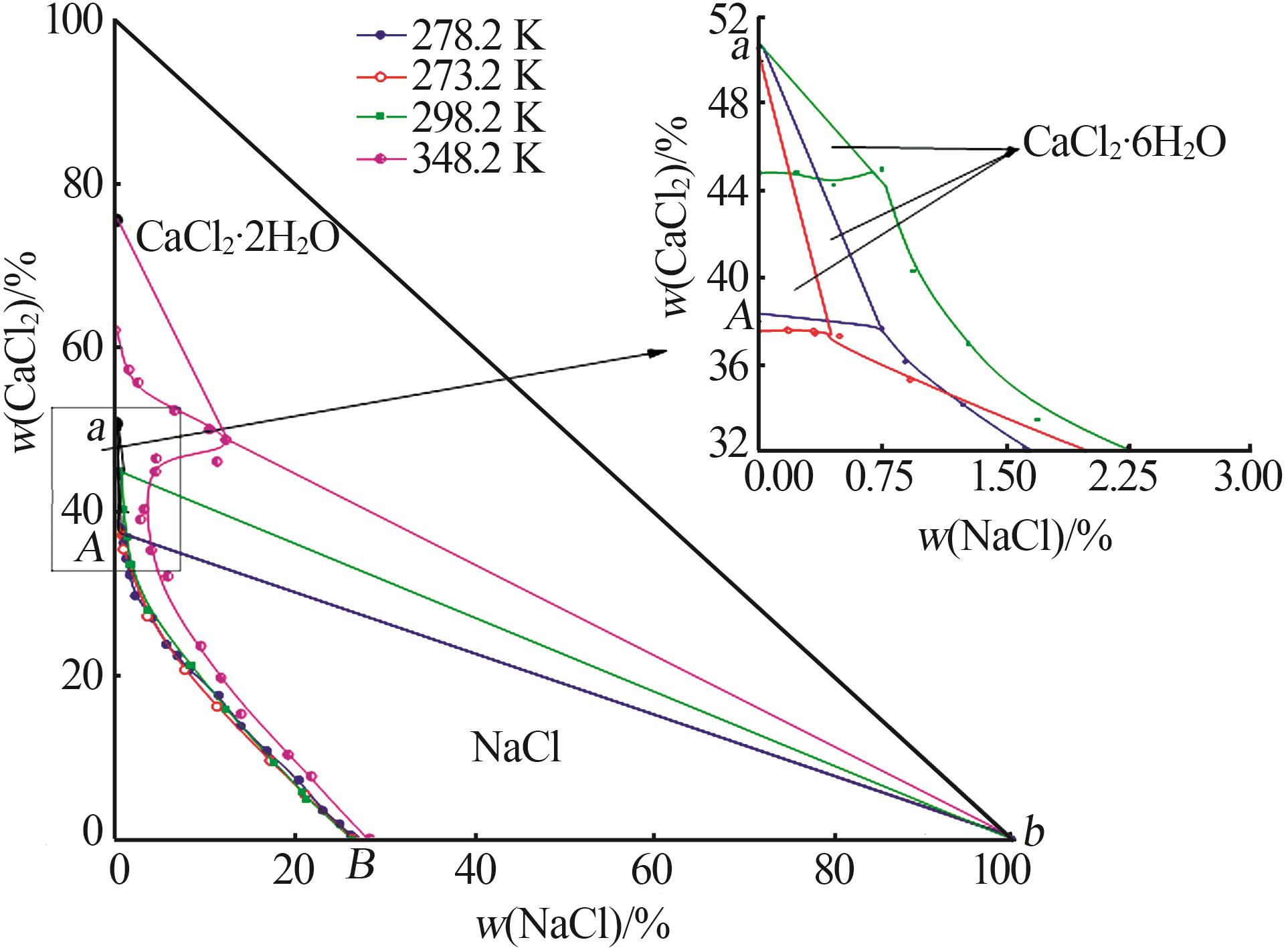
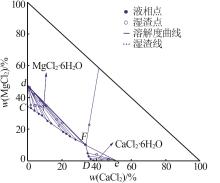
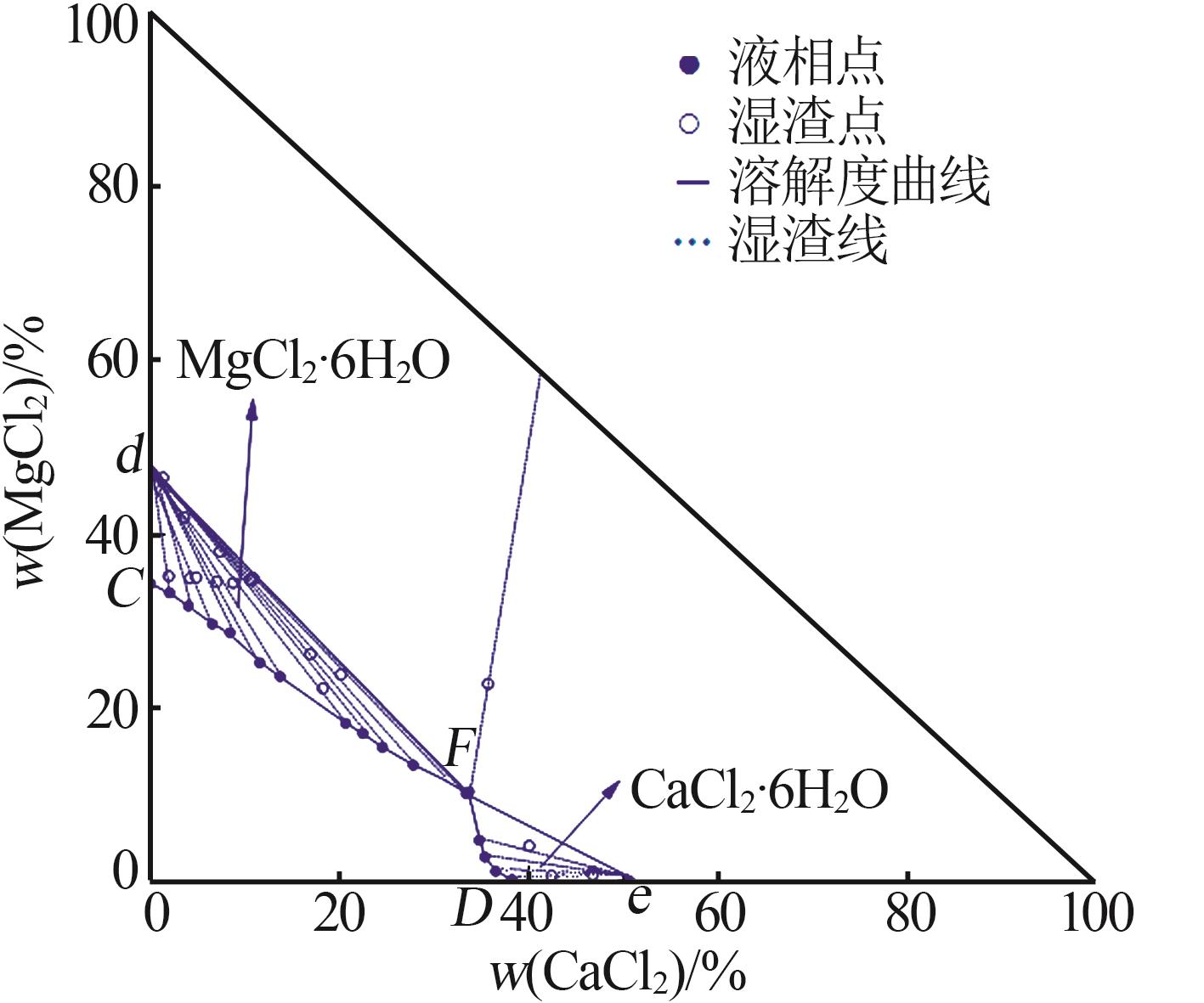
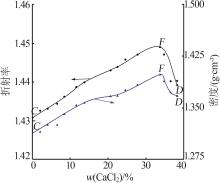
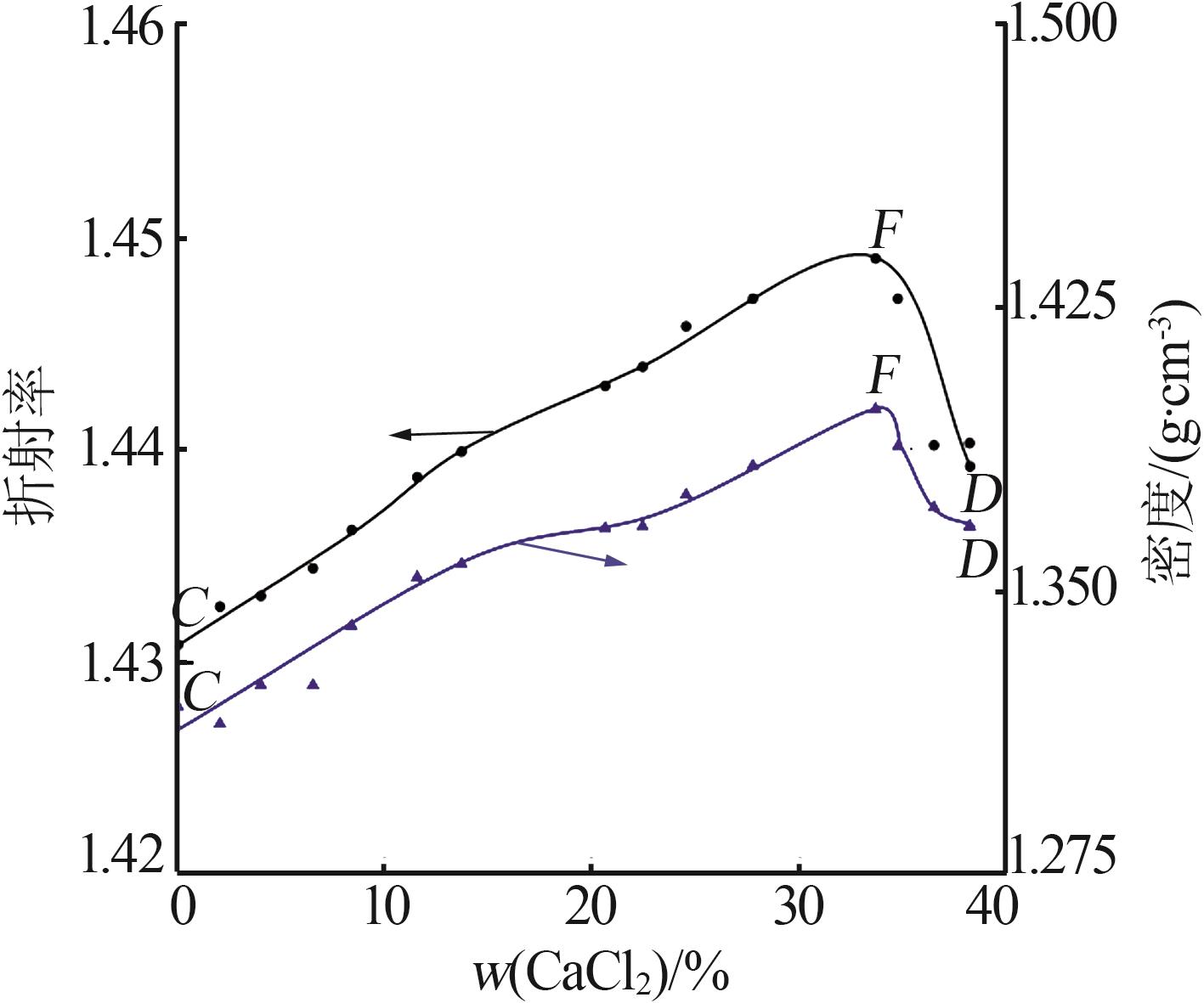
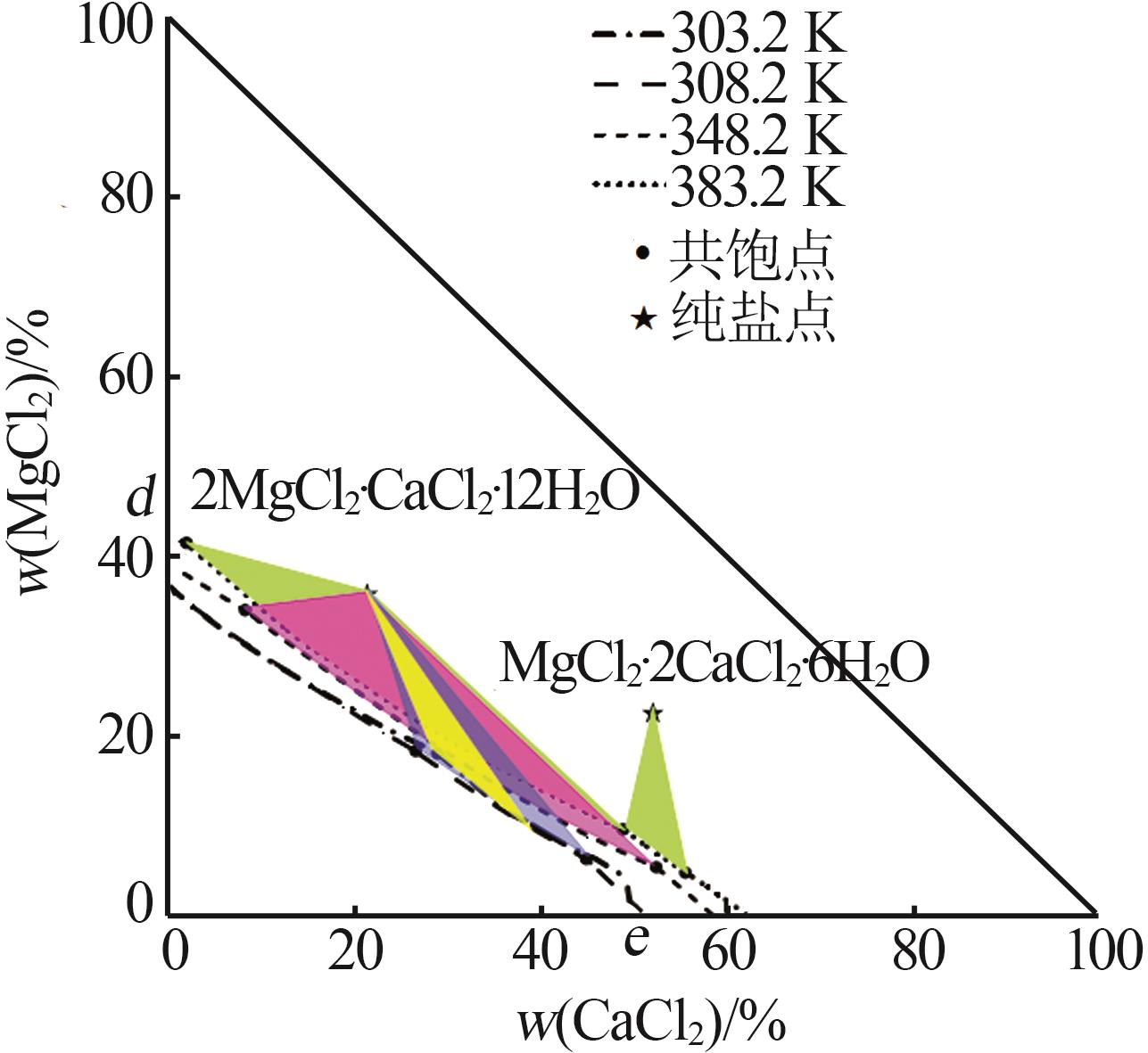
Solubility,density,and refractive index of ternary system of Na+(Mg2+),Ca2+∥Cl--H2O at 278.2 K"
序 号 | 密度/ (g·cm-3) | 折射 率 | 平衡液相 组成/% | 平衡固相 组成/% | 平衡 固相 | ||
|---|---|---|---|---|---|---|---|
| Na+,Ca2+∥Cl--H2O | |||||||
w (NaCl) | w (CaCl2) | w (NaCl) | w (CaCl2) | ||||
| 1,B | 1.194 1 | 1.381 9 | 26.30 | 0.00 | — | — | NaCl |
| 2 | 1.195 0 | 1.382 7 | 26.26 | 0.40 | 66.09 | 0.32 | NaCl |
| 3 | 1.204 8 | 1.383 9 | 25.03 | 1.79 | 99.67 | 0.25 | NaCl |
| 4 | 1.202 7 | 1.385 0 | 23.11 | 3.47 | 97.27 | 0.38 | NaCl |
| 5 | 1.210 5 | 1.389 1 | 20.48 | 7.14 | 94.25 | 1.09 | NaCl |
| 6 | 1.219 4 | 1.391 2 | 16.88 | 10.72 | 48.71 | 6.56 | NaCl |
| 7 | 1.221 3 | 1.395 2 | 13.99 | 13.75 | 41.03 | 9.50 | NaCl |
| 8 | 1.244 1 | 1.398 2 | 11.55 | 17.50 | 95.05 | 1.71 | NaCl |
| 9 | 1.254 8 | 1.404 5 | 8.21 | 20.60 | 63.08 | 8.55 | NaCl |
| 10 | 1.264 5 | 1.404 8 | 6.96 | 22.34 | 61.52 | 9.40 | NaCl |
| 11 | 1.264 0 | 1.408 6 | 5.70 | 23.72 | 91.35 | 2.98 | NaCl |
| 12 | 1.280 7 | 1.409 9 | 4.17 | 26.93 | 92.87 | 2.67 | NaCl |
| 13 | 1.297 2 | 1.415 3 | 2.23 | 29.66 | 73.64 | 7.40 | NaCl |
| 14 | 1.320 5 | 1.425 0 | 1.63 | 32.18 | 81.52 | 6.02 | NaCl |
| 15 | 1.333 0 | 1.428 2 | 1.25 | 34.16 | 57.44 | 14.88 | NaCl |
| 16 | 1.348 9 | 1.434 3 | 0.90 | 36.14 | 51.48 | 16.53 | NaCl |
| 17,E | 1.371 7 | 1.439 8 | 0.76 | 37.66 | 10.44 | 43.11 | NaCl+CaCl2· 6H2O |
| 18,A | 1.358 2 | 1.439 1 | 0.00 | 38.36 | — | — | CaCl2· 6H2O |
| Mg2+,Ca2+∥Cl--H2O | |||||||
w (CaCl2) | w (MgCl2) | w (CaCl2) | w (MgCl2) | ||||
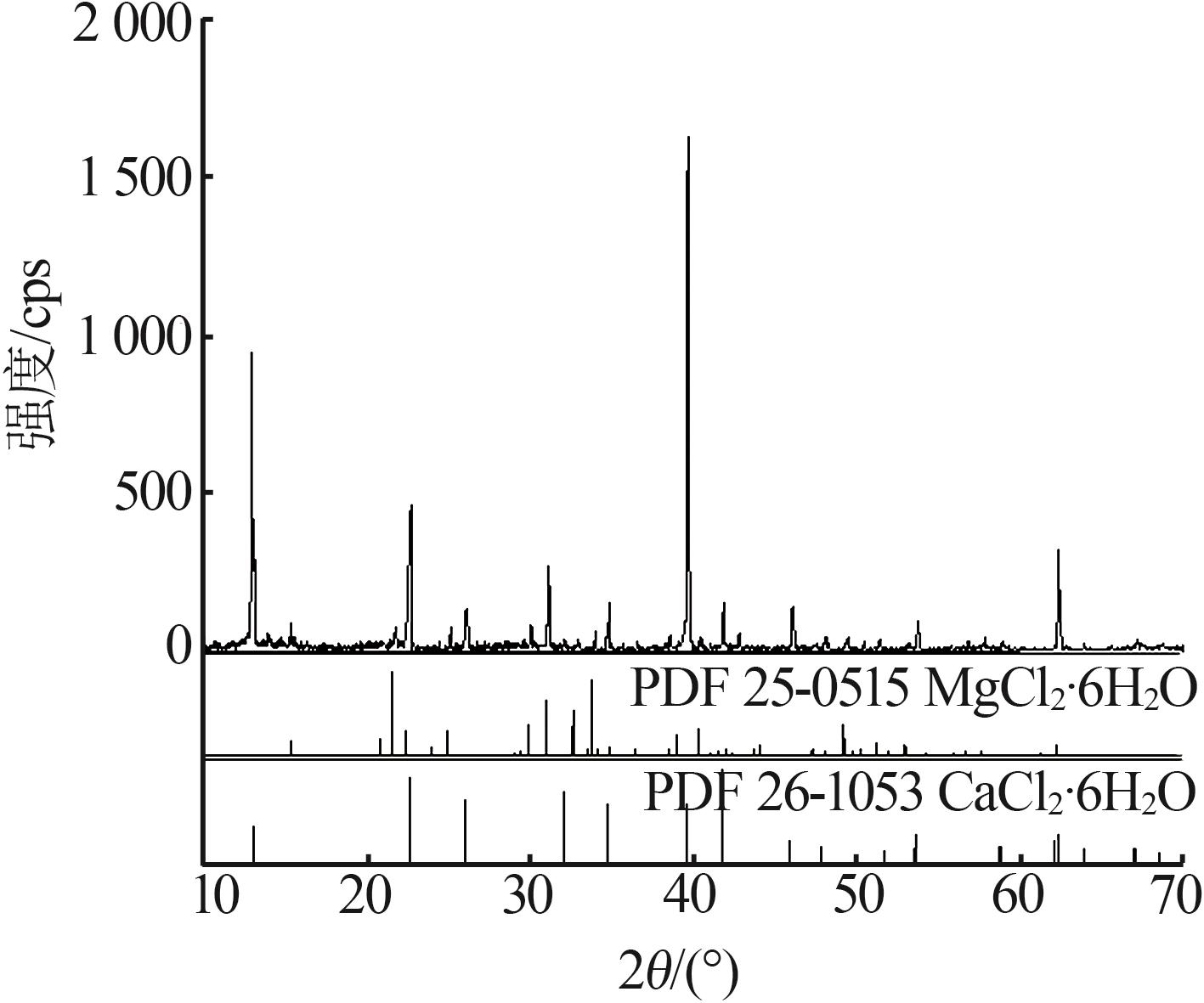
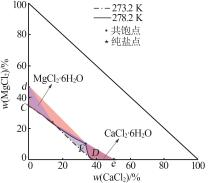
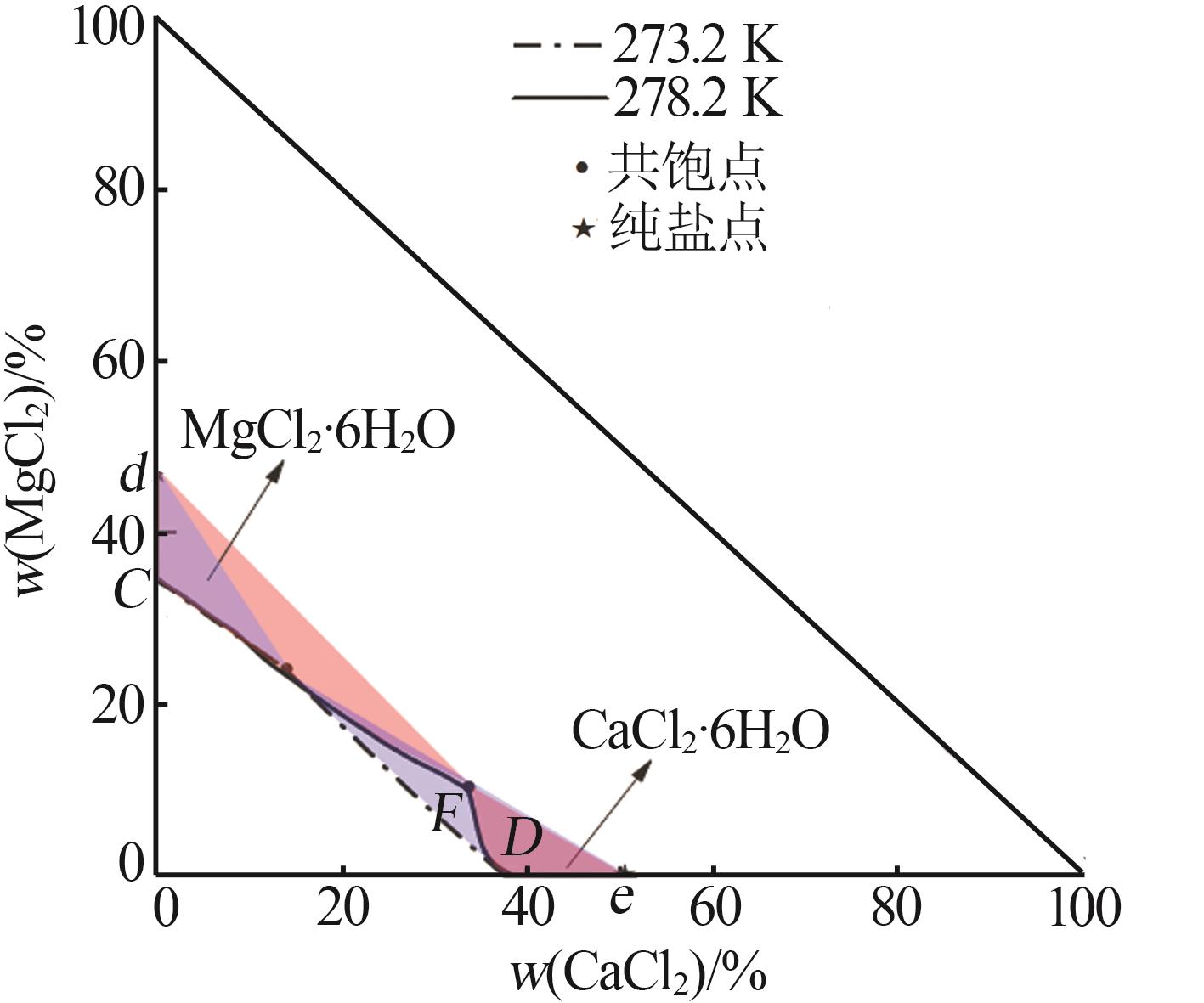
| 1,C | 1.319 3 | 1.430 8 | 0.00 | 34.21 | — | — | MgCl2· 6H2O |
| 2 | 1.314 8 | 1.432 6 | 2.02 | 33.19 | 1.93 | 35.10 | MgCl2· 6H2O |
| 3 | 1.325 0 | 1.433 1 | 4.00 | 31.68 | 3.97 | 34.86 | MgCl2· 6H2O |
| 4 | 1.325 0 | 1.434 4 | 6.53 | 29.57 | 4.84 | 34.95 | MgCl2· 6H2O |
| 5 | 1.340 7 | 1.436 2 | 8.40 | 28.59 | 7.03 | 34.46 | MgCl2· 6H2O |
| 6 | 1.353 6 | 1.438 7 | 11.57 | 25.11 | 3.56 | 41.88 | MgCl2· 6H2O |
| 7 | 1.357 1 | 1.439 9 | 13.72 | 23.53 | 1.33 | 46.45 | MgCl2· 6H2O |
| 8 | 1.366 6 | 1.443 0 | 20.67 | 18.13 | 8.74 | 34.31 | MgCl2· 6H2O |
| 9 | 1.367 1 | 1.443 9 | 22.48 | 16.97 | 16.92 | 26.08 | MgCl2· 6H2O |
| 10 | 1.375 5 | 1.445 8 | 24.58 | 15.35 | 10.87 | 34.87 | MgCl2· 6H2O |
| 11 | 1.383 2 | 1.447 1 | 27.81 | 13.30 | 20.16 | 23.76 | MgCl2· 6H2O |
| 12,F | 1.398 2 | 1.449 0 | 33.75 | 10.26 | 35.76 | 22.66 | MgCl2· 6H2O+CaCl2· 6H2O |
| 13 | 1.388 2 | 1.447 1 | 34.83 | 4.65 | 40.12 | 3.99 | CaCl2· 6H2O |
| 14 | 1.384 1 | 1.440 0 | 35.41 | 2.69 | 46.90 | 1.00 | CaCl2· 6H2O |
| 15 | 1.372 1 | 1.440 2 | 36.57 | 1.05 | 46.85 | 0.64 | CaCl2· 6H2O |
| 16 | 1.367 2 | 1.440 3 | 38.30 | 0.10 | 42.47 | 0.08 | CaCl2· 6H2O |
| 17,D | 1.366 8 | 1.439 2 | 38.32 | 0.00 | — | — | CaCl2· 6H2O |
| 1 | 张苏江,张琳,姜爱玲,等.中国盐湖资源开发利用现状与发展建议[J].无机盐工业,2022,54(10):13-21. |
| ZHANG Sujiang, ZHANG Lin, JIANG Ailing,et al.Current situation and development suggestions of development and utilization of salt lake resources in China[J].Inorganic Chemicals Industry,2022,54(10):13-21. | |
| 2 | 熊增华,王石军.察尔汗盐湖资源开发利用现状及关键技术进展[J].化工矿物与加工,2021,50(1):33-37. |
| XIONG Zenghua, WANG Shijun.Status and progress on key technologies for development and utilization of resources in Qarhan salt lake[J].Industrial Minerals & Processing,2021,50(1):33- 37. | |
| 3 | 吴朝香.盐湖尾矿高效综合利用产业化关键技术研究[J].无机盐工业,2019,51(2):40-41,71. |
| WU Zhaoxiang.Research on key technology of industrialization for high efficient comprehensive utilization of salt lake tailings[J].Inorganic Chemicals Industry,2019,51(2):40-41,71. | |
| 4 | 段雪,林彦军,项顼,等.青海盐湖镁锂资源综合利用的建议与实践[J].青海科技,2022,29(3):4-10. |
| DUAN Xue, LIN Yanjun, XIANG Xu,et al.Suggestion and practice on comprehensive utilization of magnesium and lithium resources in Qinghai salt lakes[J].Qinghai Science and Technology,2022,29(3):4-10. | |
| 5 | 刘佳,葛飞,钟永恒,等.建设世界级盐湖产业基地的战略思考[J].无机盐工业,2022,54(10):30-36. |
| LIU Jia, GE Fei, ZHONG Yongheng,et al.Strategic thinking on constructing world-class salt lake industrial base[J].Inorganic Che-Industry micals,2022,54(10):30-36. | |
| 6 | 毕秋艳,党力,曹海莲,等.青海盐湖镁资源开发与利用研究进展[J].盐湖研究,2022,30(1):101-109. |
| BI Qiuyan, DANG Li, CAO Hailian,et al.Development and utilization of magnesium resources in Qinghai salt lakes[J].Journal of Salt Lake Research,2022,30(1):101-109. | |
| 7 | YU Xudong, YAO Zhihao, ZHAO Zhixing,et al.Phase equilibria of aqueous ternary systems Li2SO4+Na2SO4+H2O and Na2SO4+K2SO4+H2O at 303.2 K[J].J.Chem.Eng.Data,2023,68(2):474-482. |
| 8 | 赵志星,姚智豪,黄琴,等.四元体系Li2SO4+Na2SO4+K2SO4+H2O298.2 K相平衡研究[J].盐湖研究,2022,30(4):41-49. |
| ZHAO Zhixing, YAO Zhihao, HUANG Qin,et al.Phase equilibria of aqueous quaternary system Li2SO4+Na2SO4+K2SO4+H2O at 298.2 K[J].Journal of Salt Lake Research,2022,30(4):41-49. | |
| 9 | 成怀刚,程芳琴.水盐体系相分离[M].北京:冶金工业出版社,2022. |
| 10 | WANG Xia, ZHAO Kaiyu, GUO Yafei,et al.Experimental determination and thermodynamic model of solid-liquid equilibria in the ternary system (LiCl+CaCl2+H2O) at 273.15 K[J].Journal of Chemical & Engineering Data,2019,64(1):249-254. |
| 11 | ZENG Dewen, XU Wenfang, VOIGT W,et al.Thermodynamic study of the system(LiCl+CaCl2+H2O)[J].The Journal of Chemical Thermodynamics,2008,40(7):1157-1165. |
| 12 | 姚智豪,孟浩,于旭东,等.三元体系KCl+CaCl2+H2O在278.2 K及308.2 K下的稳定相平衡研究[J].矿产保护与利用,2021,41(6):112-116. |
| YAO Zhihao, MENG Hao, YU Xudong,et al.Stable phase equili-bria of ternary system KCl+CaCl 2 +H2O at 278.2 K and 308.2 K[J].Conservation and Utilization of Mineral Resources,2021,41(6):112-116. | |
| 13 | IGELSRUD I, THOMPSON T G.Equilibria in the saturated solutions of salts occurring in sea water.I.The ternary systems MgCl2-KCl-H2O,MgCl2-CaCl2-H2O,CaCl2-KCl-H2O and CaCl2-NaCl-H2O at 0°[J].Journal of the American Chemical Society,1936,58:318-322. |
| 14 | LIGHTFOOT W, PRUTTON C F.Equilibria in saturated solutions;the ternary systems CaCl2-MgCl2-H2O,CaCl2-KCl-H2O,and MgCl2-KCl-H2O at 35 degrees[J].Journal of the American Chemical Society,1946,68(6):1001-1002. |
| 15 | LIGHTFOOT W J, PRUTTON C F.Equilibria in saturated salt solutions.Ⅱ.The ternary systems CaCl2-MgCl2-H2O,CaCl2-KCl-H2O and MgCl2-KCl-H2O at 75°[J].Journal of the American Chemical Society,2002,69(9):2098-2100. |
| 16 | YANG Jimin, LIU Xiaolin, LIANG Peipei.Solubilities of salts in the ternary systems NaCl+CaCl2+H2O and KCl+CaCl2+H2O at 75 ℃[J].Russian Journal of Physical Chemistry A,2011,85(7):1149-1154. |
| 17 | 丁秀萍,毕玉敬,时历杰,等.三元体系NaCl-CaCl2-H2O 25 ℃相关系研究[J].盐湖研究,2009,17(3):40-43. |
| DING Xiuping, BI Yujing, SHI Lijie,et al.Solubility in the ternary system NaCl-CaCl2-H2O at 25 ℃[J].Journal of Salt Lake Research,2009,17(3):40-43. | |
| 18 | 崔瑞芝,李武,董亚萍,等.298 K四元体系LiCl-MgCl2-CaCl2-H2O相平衡实验及溶解度计算[J].化工学报,2018,69(10):4148-4155. |
| CUI Ruizhi, LI Wu, DONG Yaping,et al.Measurements and calculations of solid-liquid equilibria in quaternary system LiCl-MgCl2-CaCl2-H2O at 298 K[J].CIESC Journal,2018,69(10):4148-4155. | |
| 19 | ASSARSSON G O.Equilibria in aqueous systems containing K+,Na+,Ca2+,Mg2+ and Cl-.Ⅲ.The ternary system CaCl2-MgCl2-H2O[J].Journal of the American Chemical Society,1950,72(4):1442-1444. |
| 20 | 李华山,张游,李飞,等.303.15 K三元体系 CaCl2-MgCl2-H2O相平衡实验及计算[J].化学工程,2022,50(8):40-45. |
| LI Huashan, ZHANG You, LI Fei,et al.Phase equilibrium experiments and calculations in ternary system CaCl2-MgCl2-H2O at 303.15 K[J].Chemical Engineering(China),2022,50(8):40-45. | |
| 21 | 中国科学院青海盐湖研究所分析室.卤水和盐的分析方法[M].2版.北京:科学出版社,1988. |
| 22 | VAN’T HOFF J H, KENRICK F B, SILCOCK H L.Solubilities of inorganic and organic compounds[M].Oxford:Pergamon Press,1979:196. |
| 23 | ZHOU Huan, GU Xiaolong, DAI Yaping,et al.Thermodynamic modeling and phase diagram prediction of salt lake brine systems.I.Aqueous Mg2+-Ca2+-Cl- binary and ternary systems[J].Chinese Journal of Chemical Engineering,2020,28(9):2391-2408. |
| [1] | YANG Yousheng, YAO Zhihao, ZHAO Zhixing, FENG Xia, ZENG Ying, YU Xudong. Research progress of lithium-rich sulfate type salt lake brine evaporation experiment [J]. Inorganic Chemicals Industry, 2024, 56(4): 1-7. |
| [2] | DENG Wenqing,ZHU Jing,FAN Xiaojuan,CHEN Yan,LI Tianxiang. Phase equilibria of ternary system of KH2PO4-(NH2)2CO-H2O at 313.15 K [J]. Inorganic Chemicals Industry, 2023, 55(1): 100-105. |
| [3] | LI Yan,WANG Yansong,CHENG Huaigang,KANG Jin,LI Enze,LÜ Hongzhou,LIU Qian. Rapid quality inspection of high-purity lithium carbonate based on determination of magnesium by water-soluble probe method [J]. Inorganic Chemicals Industry, 2023, 55(1): 87-92. |
| [4] | CHEN Shuai,YANG Bo,CHEN Niancu,LUO Jun,REN Siying,ZENG Ying,YU Xudong. Study on phase equilibria of ternary system NH4Cl+MgCl2+H2O at 323.2 K [J]. Inorganic Chemicals Industry, 2022, 54(4): 100-103. |
| [5] | MA Yujun,WANG Xiao,LI Shuya,ZHANG Fukang,LI Juan. Phase equilibria of quaternary system of NaCl+NaBO2+Na2CO3+H2O at 298.15 K [J]. Inorganic Chemicals Industry, 2022, 54(10): 42-46. |
| [6] | ZHANG Huanhuan,CHENG Zhuo,TANG Xiuhua,ZHANG Fengzhen. Determination and correlation of solubility of potassium dihydrogen phosphate in acetonitrile-water solvent [J]. Inorganic Chemicals Industry, 2022, 54(1): 83-85. |
| [7] | Wang Bin,Deng Xiaochuan,Shi Yifei,Dong Chaochao,Fan Faying,Zhu Chaoliang,Fan Jie. Online determination of the solubility of lithium carbonate in water and NaCl-KCl solution system [J]. Inorganic Chemicals Industry, 2021, 53(7): 73-79. |
| [8] | CHEN Yunfei,MAO Lili,LIU Dan,WANG Haizeng. Measurement and correlation of solubility of magnesium chloride hexahydrate in six polyols [J]. Inorganic Chemicals Industry, 2021, 53(12): 85-90. |
| [9] | JIN Fang,LI Hongpu,CHANG Donghai. Experiment on natural evaporation of brine in Nanyishan anticline structural area of Qaidam Basin [J]. Inorganic Chemicals Industry, 2021, 53(11): 86-90. |
| [10] | Yang Jiamin,Zhu Jing,Hu Xue,Wu Qiang,Li Tianxiang. Determination and correlation for solid-liquid phase equilibrium of ternary KCl-NH4Cl-H2O and KH2PO4-NH4H2PO4-H2O systems at 283.15 K [J]. Inorganic Chemicals Industry, 2021, 53(1): 30-35. |
| [11] | Liu Juexin,Zheng Chenggang,Ye Shichao. Determination and application of thermodynamic data of potassium dihydrogen phosphate crystal [J]. Inorganic Chemicals Industry, 2021, 53(1): 62-64. |
| [12] | Zhang Liyuan,Wang Gang,Qi Meiling,Xie Yulong. Effect of surfactants on metastable zone and induction period of KCl crystals in carnallite [J]. Inorganic Chemicals Industry, 2020, 52(6): 46-49. |
| [13] | Shi Xingxing,Hu Kaibao,Wang Zhanhe,Pan Jingzhong,Cui Jingui,Luo Ruiyin,Du Wei,Tang Na. Study on salt crystallization law of brine frozen mirabilite and frozen mirabilite mother liquor evaporation in Jilantai Salt Lake [J]. Inorganic Chemicals Industry, 2020, 52(6): 54-58. |
| [14] | Wang Jukui,Dong Xingfeng,Zhao Dong,Wang Shiqiang,Guo Yafei,Deng Tianlong. Solid-liquid phase equilibria in quaternary system lithium borate-potassium borate-magnesium borate-water at 308.15 K [J]. Inorganic Chemicals Industry, 2020, 52(5): 27-30. |
| [15] | Qi Meiling,Wang Gang,Zhang Liyuan,Xie Yulong. Determination of KCl solubility,interfacial region and induction period in carnallite [J]. Inorganic Chemicals Industry, 2020, 52(5): 45-49. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||