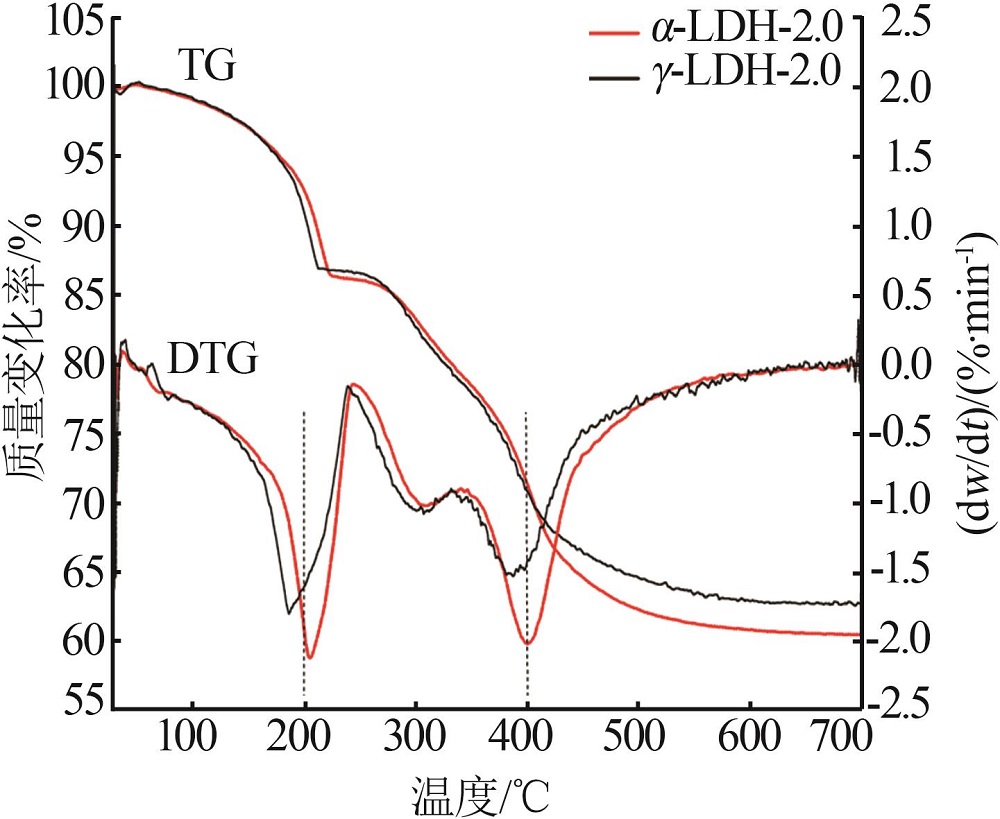
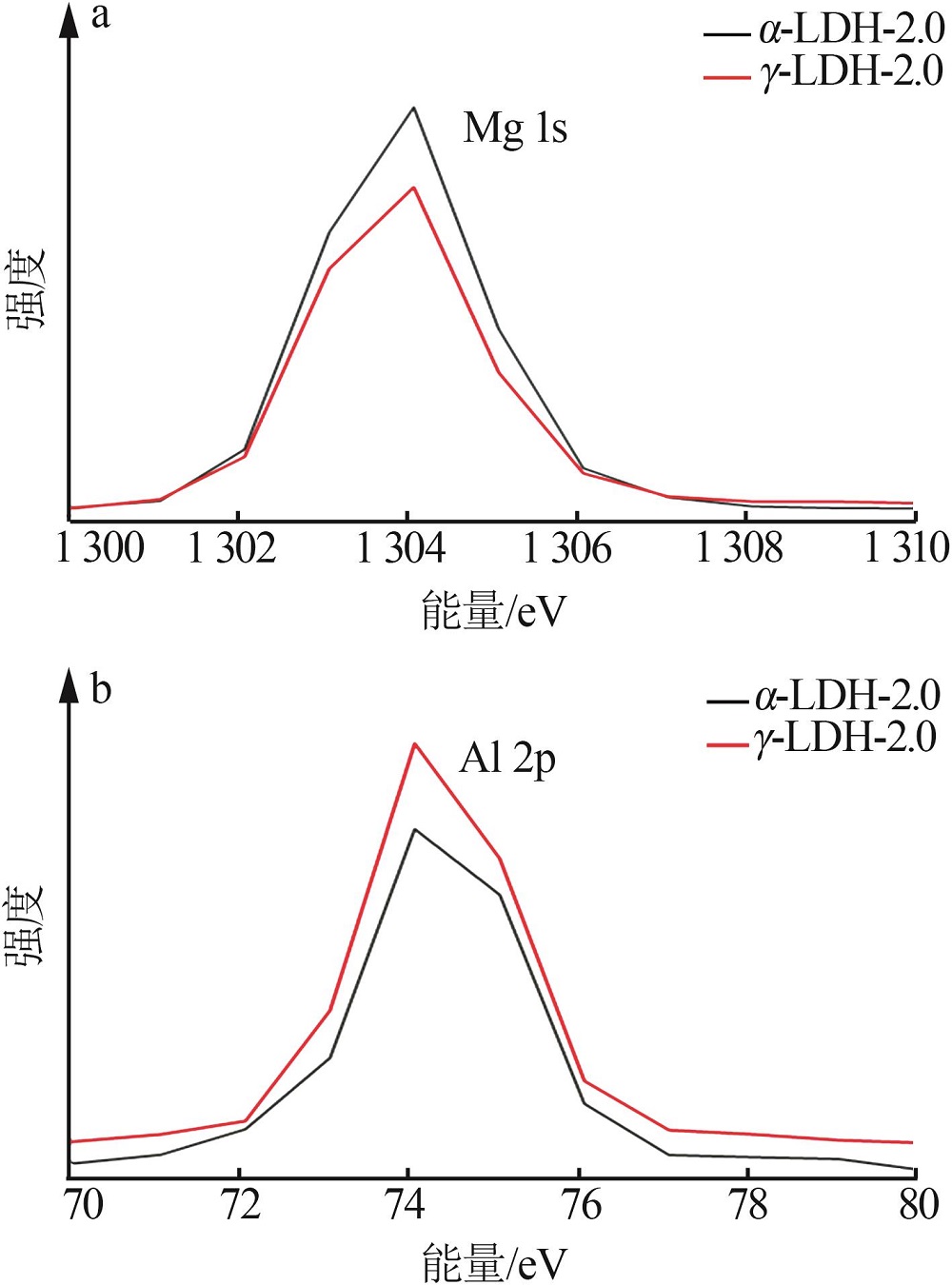
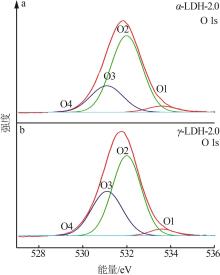
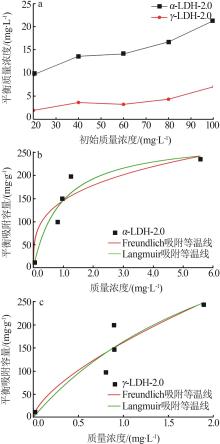
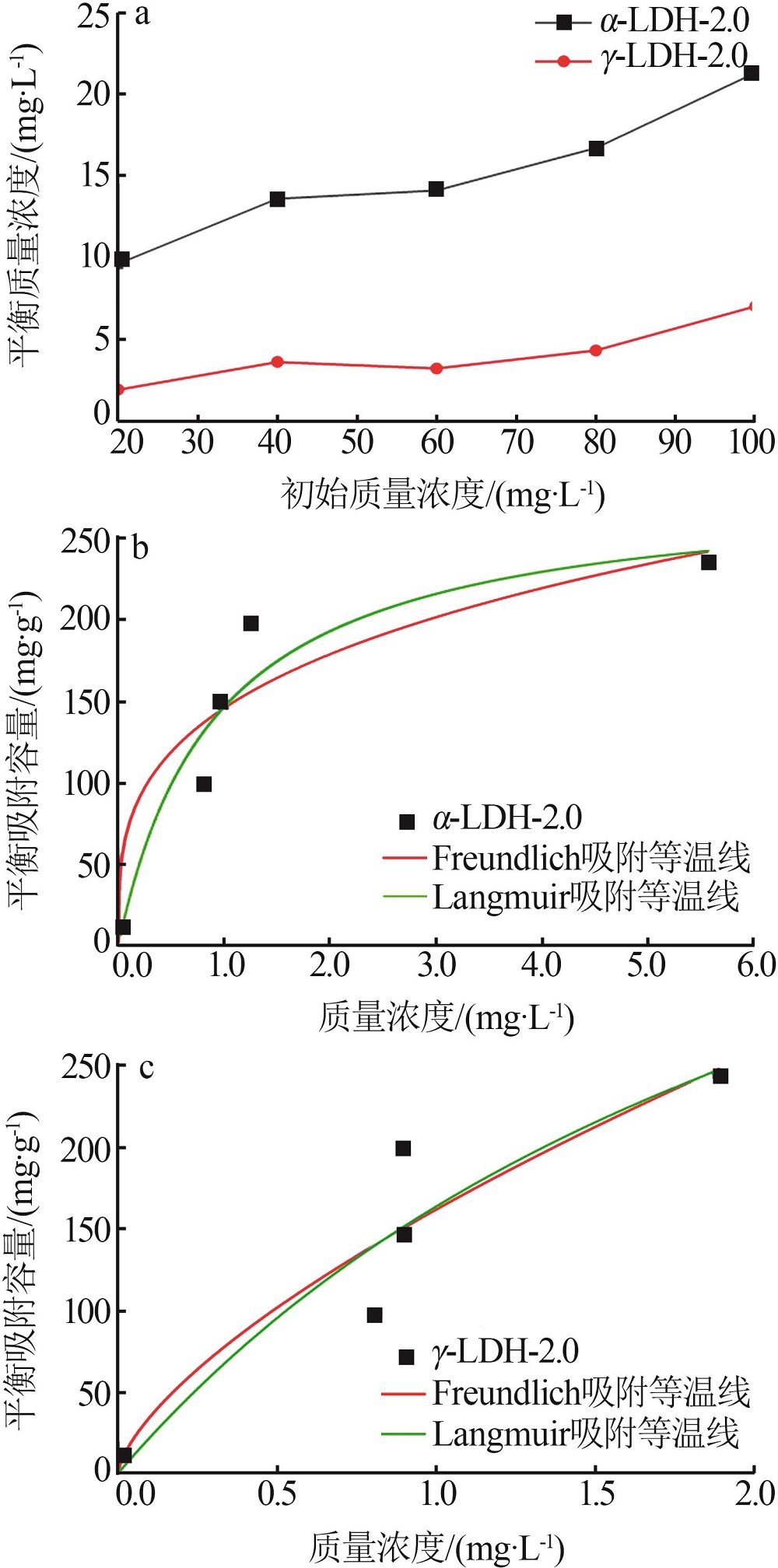
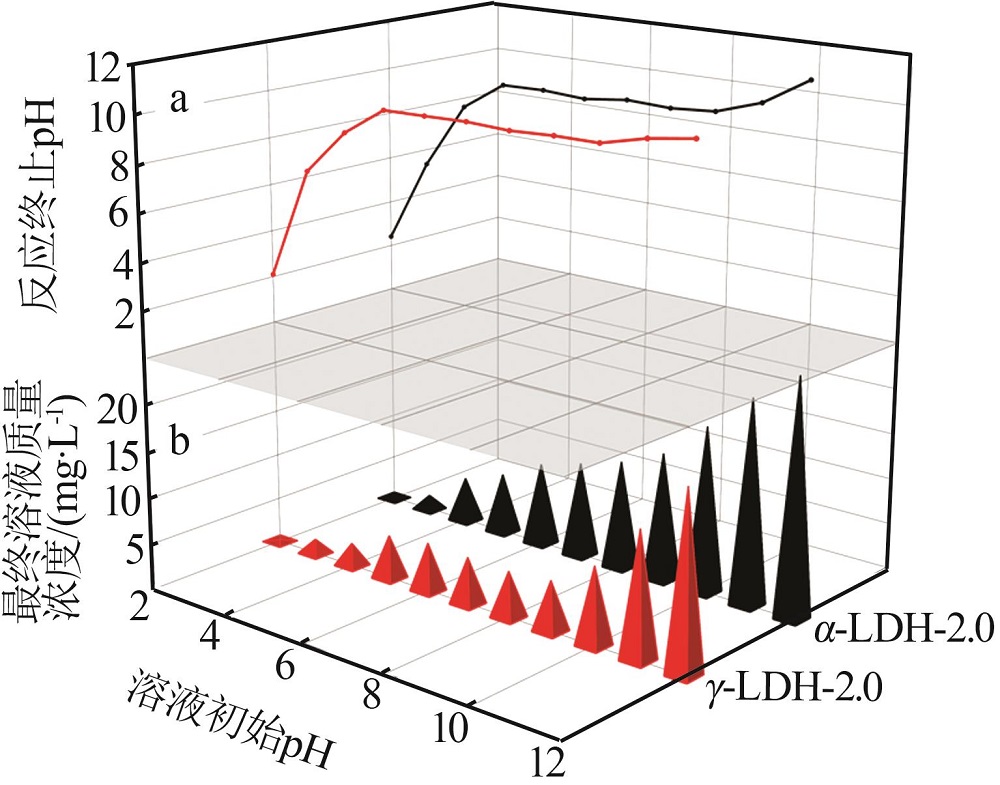
Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (12): 51-59.doi: 10.19964/j.issn.1006-4990.2022-0213
• Research & Development • Previous Articles Next Articles
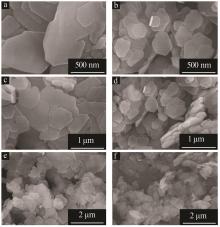
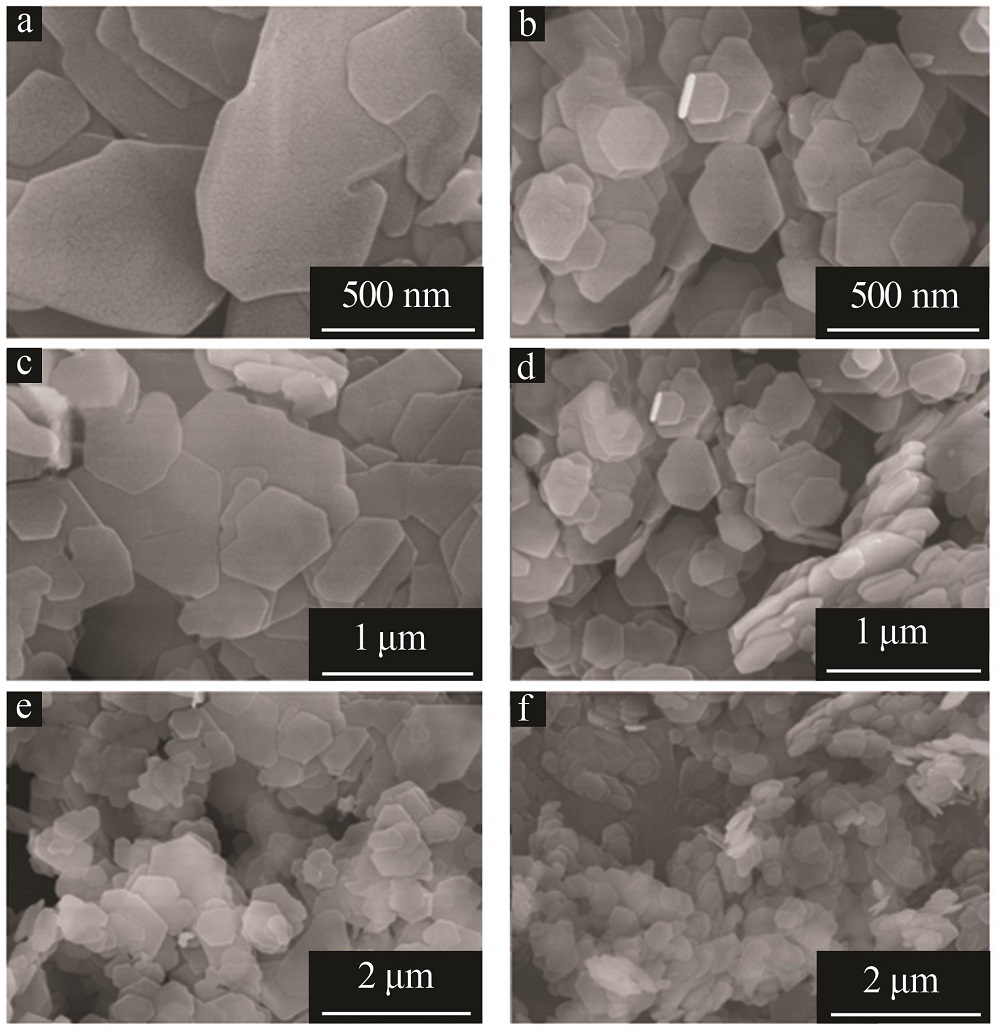
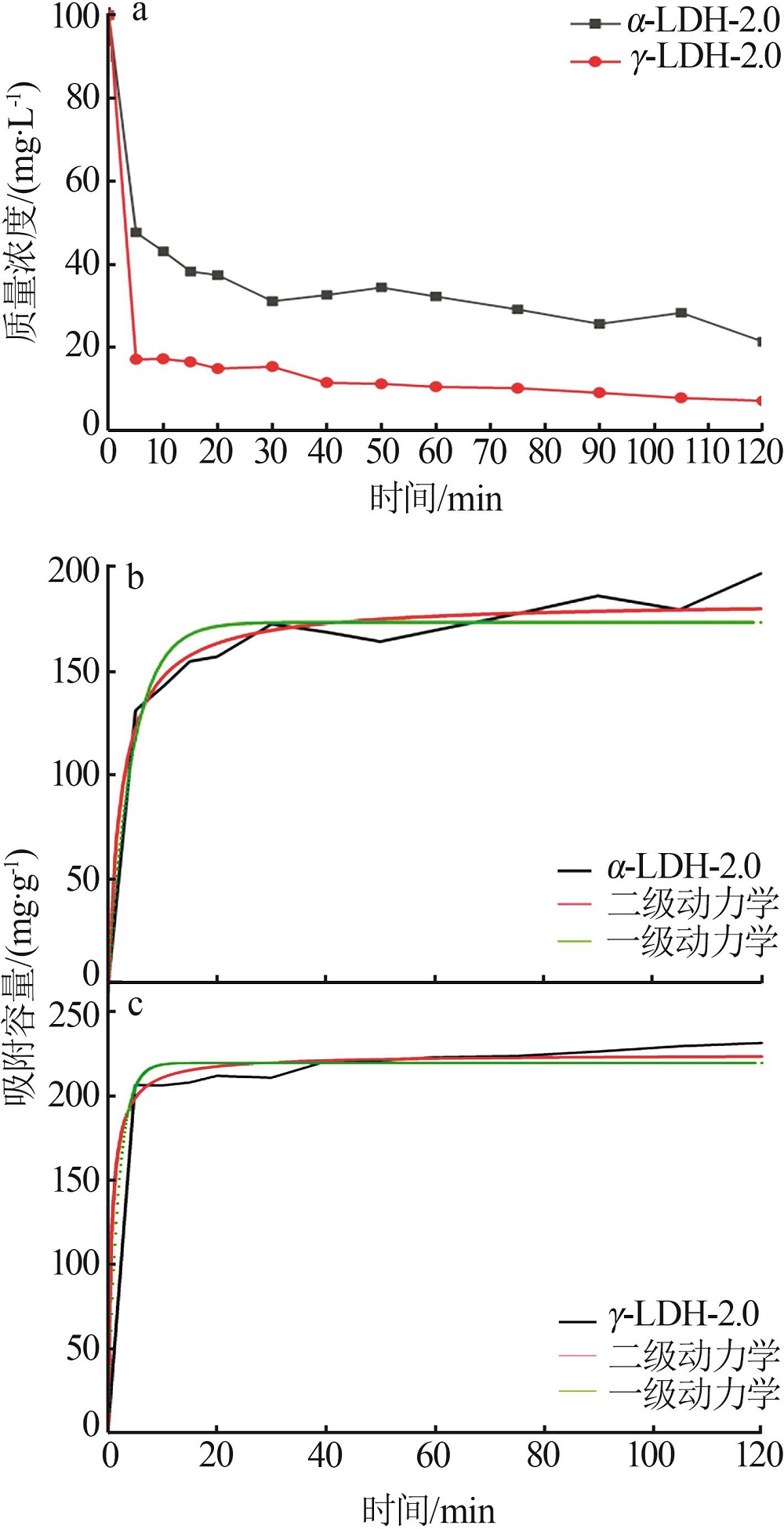
Effect of α/γ-alumina on structure and properties of synthesized Mg-Al hydrotalcite
LI Xing1( ),QIN Jun1(
),QIN Jun1( ),LU Qing1,XU Yong2,ZHANG Lijin2
),LU Qing1,XU Yong2,ZHANG Lijin2
- 1. Guizhou University,Key Laboratory of Karst Environment and Geological Hazard Prevention,Guiyang 550025,China
2. Guizhou University,School of Resources and Environmental Engineering
-
Received:2022-04-07Online:2022-12-10Published:2022-12-19 -
Contact:QIN Jun E-mail:1095147391@qq.com;qi_njun@163.com
CLC Number:
Cite this article
LI Xing,QIN Jun,LU Qing,XU Yong,ZHANG Lijin. Effect of α/γ-alumina on structure and properties of synthesized Mg-Al hydrotalcite[J]. Inorganic Chemicals Industry, 2022, 54(12): 51-59.
share this article
| 1 | 杨权成, 张开永, 郭德, 等. 介孔硅酸钙吸附亚甲基蓝性能研究[J]. 无机盐工业, 2021, 53(10):86-91. |
| YANG Quancheng, ZHANG Kaiyong, GUO De, et al. Research on adsorption properties of methylene blue by mesoporous calcium silicate[J]. Inorganic Chemicals Industry, 2021, 53(10):86-91. | |
| 2 | 李立欣, 宋志伟, 康文泽, 等. 膨胀石墨在环境保护中的应用及其发展趋势[J]. 安徽农业科学, 2015, 43(1):182-184, 281. |
| LI Lixin, SONG Zhiwei, KANG Wenze, et al. Application and development trend of expanded graphite in environmental protection[J]. Journal of Anhui Agricultural Sciences, 2015, 43(1):182-184, 281. | |
| 3 | 马万征, 李忠芳, 王艳, 等. 印染废水处理技术的现状及其发展趋势[J]. 应用化工, 2012, 41(12):2154-2155, 2159. |
| MA Wanzheng, LI Zhongfang, WANG Yan, et al. The current status and advances in treatment technologies of printing and dyeing wastewater[J]. Applied Chemical Industry, 2012, 41(12):2154-2155, 2159. | |
| 4 | 许乃才, 史丹丹. 不同晶型纳米二氧化锰制备及对亚甲基蓝吸附性能[J]. 无机盐工业, 2021, 53(3):44-47. |
| XU Naicai, SHI Dandan. Preparation of manganese dioxide nanomaterials with different crystalline and their adsorption properties to methylene blue[J]. Inorganic Chemicals Industry, 2021, 53(3):44-47. | |
| 5 | 修显凯. 膨胀石墨的制备及对孔雀石绿吸附性能的研究[D].烟台:烟台大学, 2019. |
| XIU Xiankai. Preparation of expanded graphite and its adsorption performance for malachite green[D].Yantai:Yantai University, 2019. | |
| 6 | 潘翔, 付猛. 葡萄糖/膨胀石墨复合材料对亚甲基蓝的吸附性能[J]. 材料科学与工程学报, 2019, 37(4):645-650. |
| PAN Xiang, FU Meng. Preparation and characterization of AC/EG composites and their adsorption properties for methylene blue[J]. Journal of Materials Science and Engineering, 2019, 37(4):645-650. | |
| 7 | 陈燕萌, 何春妮, 潘亭, 等. 鸡蛋壳基多孔碳酸钙对刚果红吸附特性研究[J]. 无机盐工业, 2021, 53(8):91-95. |
| CHEN Yanmeng, HE Chunni, PAN Ting, et al. Study on adsorption characteristics of multi-pore calcium carbonate made from egg shell powder for Congo red[J]. Inorganic Chemicals Industry, 2021, 53(8):91-95. | |
| 8 | 朱丹琛, 李名洁, 陈彰旭, 等. 水热法合成二氧化锰及其对染料的脱色性能研究[J]. 无机盐工业, 2021, 53(11):60-65. |
| ZHU Danchen, LI Mingjie, CHEN Zhangxu, et al. Study on hydrothermal synthesis of MnO2 and its decolorization performance on dyes[J]. Inorganic Chemicals Industry, 2021, 53(11):60-65. | |
| 9 | 徐珊, 张国春, 曹宝月. 改性膨胀石墨对Hg2+吸附性能研究[J]. 商洛学院学报, 2017, 31(4):49-53. |
| XU Shan, ZHANG Guochun, CAO Baoyue. Research of adsorption performance of Hg2+ by modified expanded graphite[J]. Journal of Shangluo University, 2017, 31(4):49-53. | |
| 10 | 吕晴, 秦军, 刘江, 等. 磷石膏制备超微细硫酸钙晶须的除磷特性研究[J]. 应用化工, 2020, 49(8):2018-2023, 2044. |
| Qing LÜ, QIN Jun, LIU Jiang, et al. Study on phosphorus removal characteristics of ultrafine calcium sulfate whiskers prepared from phosphogypsum[J]. Applied Chemical Industry, 2020, 49(8):2018-2023, 2044. | |
| 11 | HE Yinhai, LIN Hai, DONG Yingbo, et al. Preferable adsorption of phosphate using lanthanum-incorporated porous zeolite:Characteristics and mechanism[J]. Applied Surface Science, 2017, 426: 995-1004. |
| 12 | YANG Qi, WANG Xiaolin, LUO Wei, et al. Effectiveness and mechanisms of phosphate adsorption on iron-modified biochars derived from waste activated sludge[J]. Bioresource Technology, 2018, 247: 537-544. |
| 13 | BOURAIE M EL, MASOUD A A. Adsorption of phosphate ions from aqueous solution by modified bentonite with magnesium hydroxide Mg(OH)2 [J]. Applied Clay Science, 2017, 140: 157- 164. |
| 14 | 刘定鹏, 秦军, 吕晴, 等. 以粉煤灰为原料制备镁铝水滑石[J]. 硅酸盐学报, 2020, 48(8):1341-1347. |
| LIU Dingpeng, QIN Jun, LV Qing, et al. Preparation of magnesium-aluminum hydrotalcite from fly ash[J]. Journal of the Chinese Ceramic Society, 2020, 48(8):1341-1347. | |
| 15 | QIN Kun, LI Fei, XU Su, et al. Sequential removal of phosphate and cesium by using zirconium oxide:A demonstration of designing sustainable adsorbents for green water treatment[J]. Chemical Engineering Journal, 2017, 322: 275-280. |
| 16 | YE Jie, CONG Xiangna, ZHANG Panyue, et al. Interaction between phosphate and acid-activated neutralized red mud during adsorption process[J]. Applied Surface Science, 2015, 356: 128- 134. |
| 17 | 邹瑜. LDHs功能材料在建筑领域的应用研究进展[J]. 无机盐工业, 2022, 54(6):13-22. |
| ZOU Yu. Research progress on application of LDHs functional materials in field of construction[J]. Inorganic Chemicals Industry, 2022, 54(6):13-22. | |
| 18 | 童孟良, 舒均杰, 王罗强. 镁铝水滑石的制备与性能研究[J]. 无机盐工业, 2014, 46(4):22-24, 45. |
| TONG Mengliang, SHU Junjie, WANG Luoqiang. Preparation and performance study on Mg-Al-hydrotalcite[J]. Inorganic Che-Industry micals, 2014, 46(4):22-24, 45. | |
| 19 | 喻文, 傅送保. 镁铝复合氧化物负载NaAlO2催化剂制备及其催化性能研究[J]. 无机盐工业, 2020, 52(2):78-83. |
| YU Wen, FU Songbao. Synthesis of Mg-Al mixed metal oxide modified by NaAlO2 catalysts and its catalytic performance[J]. Inorganic Chemicals Industry, 2020, 52(2):78-83. | |
| 20 | 陈艳玲, 谷晓凤, 马文杰, 等. Mg(Zn)-Al(Fe)层状双氢氧化物磷酸根吸附性能研究[J]. 应用化工, 2020, 49(7):1697-1701, 1705. |
| CHEN Yanling, GU Xiaofeng, MA Wenjie, et al. Phosphate adsorption properties of Mg(Zn)-Al(Fe) layered double hydroxides[J]. Applied Chemical Industry, 2020, 49(7):1697-1701, 1705. | |
| 21 | 支景鹏, 田瑶珠, 秦军, 等. 聚丙烯/改性水滑石复合材料的等温结晶动力学[J]. 工程塑料应用, 2018, 46(8):103-109. |
| ZHI Jingpeng, TIAN Yaozhu, QIN Jun, et al. Isothermal crystallization kinetics of polypropylene/modified layered double hydroxides composites[J]. Engineering Plastics Application, 2018, 46(8):103-109. | |
| 22 | GRAND L M, PALMER S J, FROST R L. Synthesis and thermal stability of hydrotalcites based upon gallium[J]. Journal of Thermal Analysis and Calorimetry, 2010, 101(1):195-198. |
| 23 | 韩晓刚, 闵建军, 顾一飞, 等. 聚氯化铝残渣制备CaFeAl-LDO及其对甲基橙的吸附[J]. 无机盐工业, 2021, 53(10):81-85. |
| HAN Xiaogang, MIN Jianjun, GU Yifei, et al. Preparation of CaFeAl-LDO from polyaluminum chloride residue and its adsorption for methyl orange[J]. Inorganic Chemicals Industry, 2021, 53(10):81-85. | |
| 24 | 王孝华, 汤琪, 牟元华, 等. 镁铝层状双金属氢氧化物对Cu2+和Cd2+的吸收动力学研究[J]. 应用化工, 2020, 49(7):1648-1653. |
| WANG Xiaohua, TANG Qi, MU Yuanhua, et al. Kinetics of uptake of Cu2+ and Cd2+ by Mg-Al layered double hydroxides[J]. Applied Chemical Industry, 2020, 49(7):1648-1653. | |
| 25 | 吕君英, 龚凡, 郭亚平. 铜铁铝类水滑石及其复合氧化物的制备与表征[J]. 无机盐工业, 2006, 38(1):20-22. |
| Junying LÜ, GONG Fan, GUO Yaping. Preparation and characterization of CuFeAl-CO3 hydrotalcite and its compounded metal oxides[J]. Inorganic Chemicals Industry, 2006, 38(1):20-22. | |
| 26 | 陈立谦, 韩冰, 刘琦. 镁铝及镁锌铝水滑石的合成与表征[J]. 无机盐工业, 2011, 43(12):38-41. |
| CHEN Liqian, HAN Bing, LIU Qi. Synthesis and characterization of Mg/Al and Mg/Zn/Al hydrotalcite[J]. Inorganic Chemicals Industry, 2011, 43(12):38-41. | |
| 27 | 王惠娟, 王春玉, 张琼. 二维层状纳米片材料制备及在电解水中应用的研究进展[J]. 无机盐工业, 2021, 53(11):25-29, 41. |
| WANG Huijuan, WANG Chunyu, ZHANG Qiong. Research progress on the preparation of two-dimensional layered nanosheet materials and its application in electrolyzed water[J]. Inorganic Chemicals Industry, 2021, 53(11):25-29, 41. | |
| 28 | 吕品, 施春辉, 李伟, 等. 镁铝水滑石的水热合成及表征[J]. 无机盐工业, 2019, 51(6):29-33, 71. |
| Pin LÜ, SHI Chunhui, LI Wei, et al. Controllable synthesis and characterization of thermal stabilizer Mg-Al-LDHs[J]. Inorganic Chemicals Industry, 2019, 51(6):29-33, 71. | |
| 29 | XU Zhiping, LU Guoqing. Hydrothermal synthesis of layered double hydroxides(LDHs) from mixed MgO and Al2O3: LDH formation mechanism[J]. Chemistry of Materials, 2005, 17(5):1055-1062. |
| 30 | LIAO Libing, ZHAO Ning, XIA Zhiguo. Hydrothermal synthesis of Mg-Al layered double hydroxides(LDHs) from natural brucite and Al(OH)3 [J]. Materials Research Bulletin, 2012, 47(11):3897-3901. |
| 31 | 韩青, 罗康碧, 李沪萍, 等. 磷石膏的高效利用:硫酸钙晶须的制备与应用[J]. 无机盐工业, 2013, 45(7):46-48. |
| HAN Qing, LUO Kangbi, LI Huping, et al. High efficient utilization of phosphogypsum:Preparation and application of calcium sulfate whiskers[J]. Inorganic Chemicals Industry, 2013, 45(7):46-48. | |
| 32 | 余家国, 徐静. 一种刚果红染料吸附剂、制备和利用其处理刚果红染料废水的方法:中国, 105817211B[P]. 2019-04-30. |
| [1] | LI Keke, XUE Jiangwei, WANG Luwei, GUAN Xuemao. Effect of MgAl-layered double hydroxides on properties of high-iron low-calcium portland cement [J]. Inorganic Chemicals Industry, 2024, 56(4): 57-63. |
| [2] | XU Mengyao, ZHANG Xin, HE Kunpeng, HE Jian, JIANG Wei. Preparation of yttrium oxide and zirconium phosphate adsorbents from zirconium-yttrium waste and evaluation of their performance [J]. Inorganic Chemicals Industry, 2024, 56(3): 116-124. |
| [3] | LI Qiaoyun, HUANG Xiuxing, WEI Wenye, CHEN Zhen. Study on adsorption of methylene blue by activated carbon with acid/alkali synergistically modified fly ash [J]. Inorganic Chemicals Industry, 2024, 56(3): 131-136. |
| [4] | LI Yang, LOU Feijian, SUI Xin, LI Keyan, LIU Fei, GUO Xinwen. Preparation of amine-functionalized fumed SiO2 materials and their performance for CO2 adsorption [J]. Inorganic Chemicals Industry, 2024, 56(2): 38-43. |
| [5] | ZHANG Li, ZHANG Dan, PAN Hongyan, DONG Yonggang, LI Wenfei, QIN Hong. Study on preparation of low ash activated carbon by phosphoric acid method [J]. Inorganic Chemicals Industry, 2024, 56(2): 95-103. |
| [6] | YAN Zhen, QIU Zhaofu, JIN Xibiao, WANG Yuan, LIU Chang, YANG Ji. Study on 4A molecular sieve loaded with Ce and γ-Fe2O3 for removal of Sb(Ⅲ) and Sb(Ⅴ) in water [J]. Inorganic Chemicals Industry, 2024, 56(1): 81-89. |
| [7] | ZHOU Shiqi, WANG Tao, JING Fangli, LUO Shizhong. Study on performance of magnesium nitrate-modified carbon molecular sieve for separation of nitrogen/methane [J]. Inorganic Chemicals Industry, 2023, 55(9): 75-80. |
| [8] | LI Chao, WANG Liping, DAI Yin, GAO Guimei, ZHANG Yunfeng, HONG Yu, XU Lijun, CUI Yongjie. Study on alkali fusion hydrothermal synthesis of 13X zeolite from high silicon tailings and its adsorption on lead,copper and zinc ions [J]. Inorganic Chemicals Industry, 2023, 55(9): 88-93. |
| [9] | WANG Yingnan, SHENG Linlin, HUANG Juan, HUANG Zhanbin. Study on adsorption performance of lead from water by coal-fired slag [J]. Inorganic Chemicals Industry, 2023, 55(8): 109-115. |
| [10] | TANG Yifu, CAO Changchun, LÜ Peng. Study on adsorption of cadmium-humic acid by hydroxyiron oxide [J]. Inorganic Chemicals Industry, 2023, 55(8): 124-131. |
| [11] | HAN Hongjing, ZHANG Jingze, LAMAO Zhuoma, HAN Jizhe, WU Yongmin, TANG Weiping. Preparation of Al-Co co-doped lithium manganese oxide and its adsorption performance of lithium [J]. Inorganic Chemicals Industry, 2023, 55(7): 38-44. |
| [12] | FAN Fangfang, TONG Zhongkai, ZUO Weiyuan. Study on adsorption of tetracycline from wastewater by calcium modified peanut shell biochar [J]. Inorganic Chemicals Industry, 2023, 55(6): 109-115. |
| [13] | TIAN Yuling, CHENG Yang, HAN Rong, ZHOU Mei, WANG Chengjie, GE Qiangru. Effect of CaO on heavy metals stability and adsorption properties of sludge-derived biochar [J]. Inorganic Chemicals Industry, 2023, 55(6): 124-129. |
| [14] | FU Minglian, CEN Jianmei, CHEN Zhangxu. Study on preparation of magnetic SiO2/chitosan composite aerogel and its adsorption for Cu2+ [J]. Inorganic Chemicals Industry, 2023, 55(6): 70-77. |
| [15] | XU Chunhui, WANG Feng, LING Changjian, WANG Zirui, TANG Zhongfeng. Research progress of CO2 capture by metal oxides modified by molten salts [J]. Inorganic Chemicals Industry, 2023, 55(5): 1-7. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||