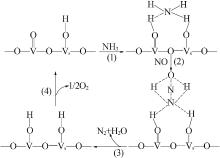
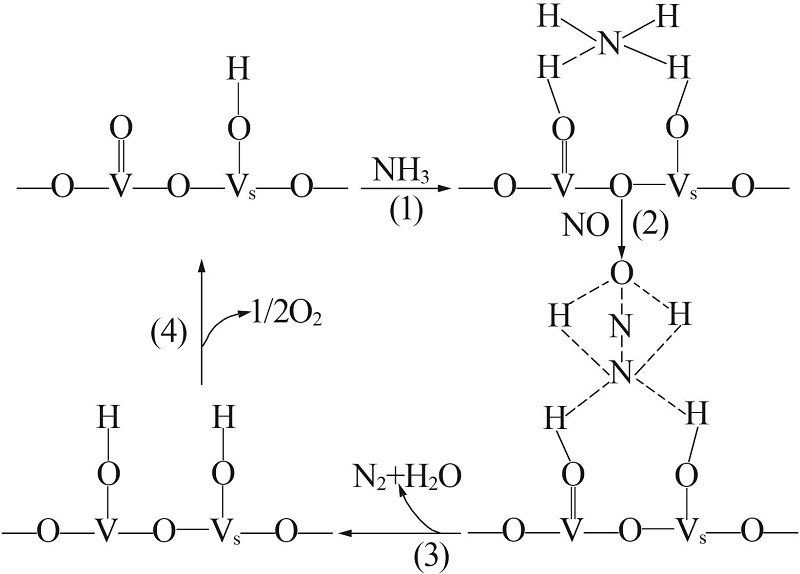
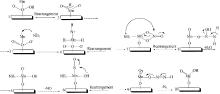
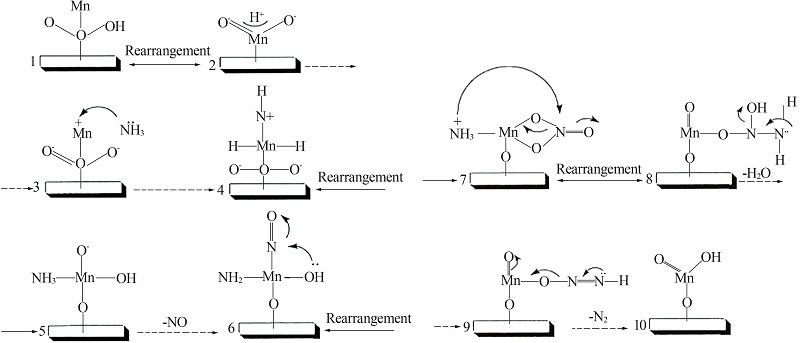
Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (11): 18-24.doi: 10.19964/j.issn.1006-4990.2021-0735
• Reviews and Special Topics • Previous Articles Next Articles
Current situation of NH3-SCR denitration catalyst and research progress on poisoning and deactivation
LI Kejun( ),LI Fangqin(
),LI Fangqin( ),REN Jianxing,LIU Xin,CHEN Linfeng,CAI Jianming
),REN Jianxing,LIU Xin,CHEN Linfeng,CAI Jianming
- College of Energy and Mechanical Engineering,Shanghai University of Electric Power,Shanghai 200090,China
-
Received:2021-12-06Online:2022-11-10Published:2022-11-23 -
Contact:LI Fangqin E-mail:1018714206@qq.com;lifangqin@shiep.edu.cn
CLC Number:
Cite this article
LI Kejun,LI Fangqin,REN Jianxing,LIU Xin,CHEN Linfeng,CAI Jianming. Current situation of NH3-SCR denitration catalyst and research progress on poisoning and deactivation[J]. Inorganic Chemicals Industry, 2022, 54(11): 18-24.
share this article
Table 1
Comparison of industrial catalysts in use and modified catalysts"
| 催化剂 | 反应条件 | NO x 最佳转化率 |
|---|---|---|
| V-W-Ti商品催化剂[ | n(NH3)∶n(NO)=1,5% O2,24 000 h-1 | 93.4%(380 ℃) |
| 1% V2O5-10% WO3/ TiO2[ | 1×10-3 NH3,1×10-3 NO,5% O2,24 000 h-1 | 86.98%(370 ℃) |
| MnO2-CeO2纳米球[ | 5×10-4 NH3,5×10-4 NO,5% O2,1×10-4 SO2,60 000 mL/(g·h) | >97%(120~250 ℃) |
| MnO2-PVP(200)[ | 5×10-4 NH3,5×10-4 NO,5% O2,50 000 h-1 | 98%(160 ℃) |
| Ce/Ti-Si(3∶1) [ | 5×10-4 NH3,5×10-4 NO,3% O2,28 000 h-1 | 90%(250~450 ℃) |
| MnO x /TiO2[ | 5×10-4 NH3,5×10-4 NO,5% O2,24 000 h-1 | 98%(200 ℃) |
| CuNd/SAPO-34[ | 2×10-4 NH3,2×10-4 NO,10% O2,5% H2O,40 000 h-1 | 86%(200 ℃) |
| 6%V2O5-9%WO3/TiO2[ | 7×10-4 NH3,7×10-4 NO,5% O2,1×10-3 SO2,129 670 h-1 | 95%~97%(220~300 ℃) |
| (Cu x Mn3-x )1-δ O4[ | 5×10-4 NH3,5×10-4 NO,3% O2,5% H2O,5×10-4 SO2,100 000 h-1 | 80%~90%(150~200 ℃) |
| 1 | 葛挺.“十四五”期间火电技术发展方向分析[J].河南电力,2020(S2):1-3. |
| GE Ting.Analysis of the development direction of thermal power technology during the“14th Five-Year Plan” period[J].Henan Electric Power,2020(S2):1-3. | |
| 2 | 姚小江,张雷,孔婷婷,等.低温氨-选择性催化还原氮氧化物催化剂的研究进展[J].工业催化,2016,24(6):1-9. |
| YAO Xiaojiang, ZHANG Lei, KONG Tingting, et al.Research progress in selective catalytic reduction of nitrogen oxides by ammonia at low temperature[J].Industrial Catalysis,2016,24(6):1-9. | |
| 3 | 王金玉.掺杂Ce对Mn基脱硝催化剂抗水抗硫性的实验研究和模拟分析[D].青岛:青岛大学,2020. |
| WANG Jinyu.Experimental study and simulation analysis on water and sulfur resistance of Mn based denitration catalyst doped with Ce[D].Qingdao:Qingdao University,2020. | |
| 4 | HAN Lupeng, CAI Sixiang, GAO Min, et al.Selective catalytic reduction of NO x with NH3 by using novel catalysts:State of the art and future prospects[J].Chemical Reviews,2019,119(19):10916-10976. |
| 5 | ODRIOZOLA J A, HEINEMANN H, SOMORJAI G A, et al.AES and TDS study of the adsorption of NH3 and NO on V2O5 and TiO2 surfaces:Mechanistic implications[J].Journal of Catalysis,1989,119(1):71-82. |
| 6 | PENG Yue, LI Junhua, SI Wenzhe, et al.Deactivation and regeneration of a commercial SCR catalyst:Comparison with alkali metals and arsenic[J].Applied Catalysis B:Environmental,2015,168/169: 195-202. |
| 7 | LARRUBIA M A, RAMIS G, BUSCA G.An FT-IR study of the adsorption of urea and ammonia over V2O5-MoO3-TiO2 SCR catalysts[J].Applied Catalysis B:Environmental,2000,27(3):L145-L151. |
| 8 | 梁冉冉.SCR催化剂脱硝及中毒机理的量子化学研究[D].北京:华北电力大学,2017. |
| LIANG Ranran.Quantum chemistry research on DeNO x and poisoning mechanism of SCR catalyst[D].Beijing:North China Electric Power University,2017. | |
| 9 | OZKAN U S, CAI Yeping, KUMTHEKAR M W.Effect of crystal morphology in selective catalytic reduction of nitric oxide over V2O5 catalysts[J].Applied Catalysis A:General,1993,96(2):365-381. |
| 10 | 刘福东,单文坡,石晓燕,等.用于NH3选择性催化还原NO的非钒基催化剂研究进展[J].催化学报,2011,32(7):1113-1128. |
| LIU Fudong, SHAN Wenpo, SHI Xiaoyan, et al.Research progress in vanadium-free catalysts for the selective catalytic reduction of NO with NH3 [J].Chinese Journal of Catalysis,2011,32(7):1113-1128. | |
| 11 |
LIU Zhong, HAN Jian, ZHAO Li, et al.Effects of Se and SeO2 on the denitrification performance of V2O5-WO3/TiO2 SCR cataly-st[J].Applied Catalysis A:General,2019,587.Doi:10.1016/j.apcata.2019.117263.
doi: 10.1016/j.apcata.2019.117263 |
| 12 | XU Junqiang, CHEN Guorong, GUO Fang, et al.Development of wide-temperature vanadium-based catalysts for selective catalytic reducing of NO x with ammonia:Review[J].Chemical Engineering Journal,2018,353: 507-518. |
| 13 |
LIU Guangli, HAN Dongtai, CHENG Jie, et al.Performance of C2H4 reductant in activated-carbon-supported MnO x -based SCR catalyst at low temperatures[J].Energies,2018,12(1).Doi:10.3390/en12010123.
doi: 10.3390/en12010123 |
| 14 | 许硕.锰系催化剂的制备及其低温SCR脱硝性能的研究[D].哈尔滨:哈尔滨工程大学,2016. |
| XU Shuo.Research on Mn-based series catalysts and mechanism for selective catalytic reduction of NO x at low-temperature[D].Harbin:Harbin Engineering University,2016. | |
| 15 | KAPTEIJN F, SINGOREDJO L, ANDREINI A, et al.Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia[J].Applied Catalysis B:Environmental,1994,3(2/3):173-189. |
| 16 | THIRUPATHI B, SMIRNIOTIS P G.Nickel-doped Mn/TiO2 as an efficient catalyst for the low-temperature SCR of NO with NH3:Catalytic evaluation and characterizations[J].Journal of Catalysis,2012,288: 74-83. |
| 17 | LI Lulu, SUN Bowen, SUN Jingfang, et al.Novel MnO x -CeO2 nanosphere catalyst for low-temperature NH3-SCR[J].Catalysis Communications,2017,100: 98-102. |
| 18 | JIANG Haoxi, WANG Yaodong, ZHOU Jiali, et al.Morphology control of manganese-based catalysts for low-temperature selective catalytic reduction of NO x [J].Materials Letters,2018,233: 250-253. |
| 19 | 牟金成.新型铁基催化剂的制备及其低温NH3选择性催化还原NO x 研究[D].大连:大连理工大学,2020. |
| MOU Jincheng.Investigation on the preparation and application in low-temperature selective catalytic reduction of NO x with NH3 over novel Fe-based catalysts[D].Dalian:Dalian University of Technology,2020. | |
| 20 | SHAVEL A, LIZ-MARZÁN L M.Shape control of iron oxide nanoparticles[J].Physical Chemistry Chemical Physics:PCCP,2009,11(19):3762-3766. |
| 21 | YANG Xingye, LI Bin, SUN Liang, et al.Effect of surface structure of α-Fe2O3 on the selective catalytic reduction of NO by NH3 [J].物理化学学报,2012,28(1):184-188. |
| YANG Xingye, LI Bin, SUN Liang, et al.Effect of surface structure of α-Fe2O3 on the selective catalytic reduction of NO by NH3 [J].Acta Physico-Chimica Sinica,2012,28(1):184-188. | |
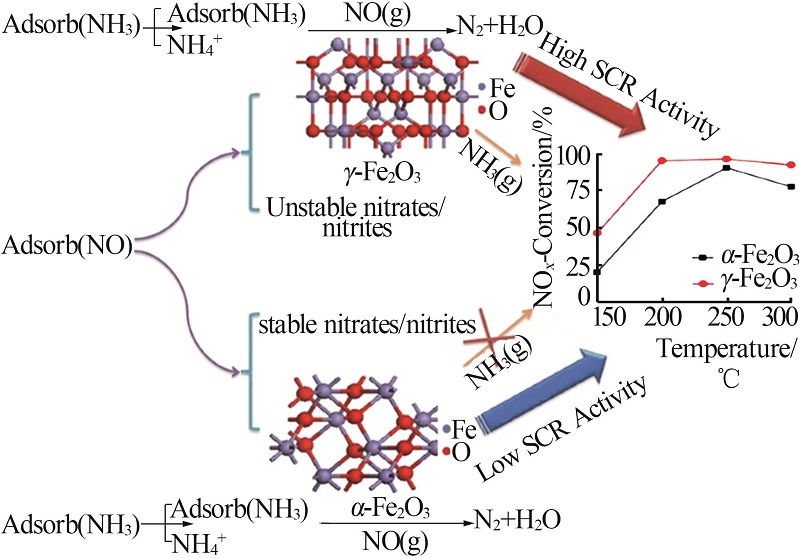
| 22 | LIU Caixia, YANG Shijian, MA Lei, et al.Comparison on the performance of α-Fe2O3 and γ-Fe2O3 for selective catalytic reduction of nitrogen oxides with ammonia[J].Catalysis Letters,2013,143(7):697-704. |
| 23 | 余雅昕.铁基NH3-SCR催化剂的低温脱硝性能及抗SO2中毒机制研究[D].南京:南京大学,2020. |
| YU Yaxin.Study on the mechanism of SO2 resistance and the catalytic performance in low-temperature selective catalytic reduction of NO by NH3 over Fe-based catalysts[D].Nanjing:Nanjing University,2020. | |
| 24 |
YANG Weijie, REN Jianuo, ZHANG Hanwen, et al.Single-atom iron as a promising low-temperature catalyst for selective catalytic reduction of NO x with NH3:A theoretical prediction[J].Fuel,2021,302.Doi:10.1016/j.fuel.2021.121041.
doi: 10.1016/j.fuel.2021.121041 |
| 25 | HE Chuan, SHEN Boxiong, CHEN Jianhong, et al.Adsorption and oxidation of elemental mercury over Ce-MnO x /Ti-PILCs[J].Environmental Science & Technology,2014,48(14):7891-7898. |
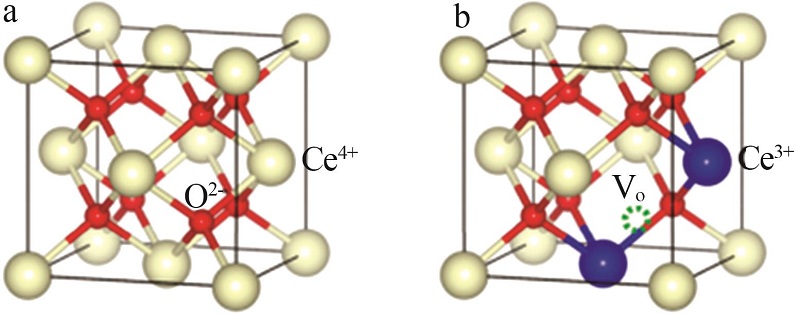
| 26 | ZHOU Jue, GUO Ruitang, ZHANG Xinfu, et al.Cerium oxide-based catalysts for low-temperature selective catalytic reduction of NO x with NH3:A review[J].Energy & Fuels,2021,35(4):2981-2998. |
| 27 |
SHAN Ye, LIU Yangxian, LI Ying, et al.A review on application of cerium-based oxides in gaseous pollutant purification[J].Separation and Purification Technology,2020,250.Doi:10.1016/j.seppur.2020.117181.
doi: 10.1016/j.seppur.2020.117181 |
| 28 | PEYROVI P, GILLOT S, DACQUIN J P, et al.The activity of CeVO4-based catalysts for ammonia-SCR:Impact of surface cerium enrichment[J].Catalysis Letters,2021,151(4):1003-1012. |
| 29 | CHEN Chuanmin, CAO Yue, LIU Songtao, et al.Review on the latest developments in modified vanadium-titanium-based SCR catalysts[J].Chinese Journal of Catalysis,2018,39(8):1347-1365. |
| 30 | LIU Caixia, CHEN Liang, LI Junhua, et al.Enhancement of activity and sulfur resistance of CeO2 supported on TiO2-SiO2 for the selective catalytic reduction of NO by NH3 [J].Environmental Science & Technology,2012,46(11):6182-6189. |
| 31 | Jinsun CHA, CHOI J C, KO J H, et al.The low-temperature SCR of NO over rice straw and sewage sludge derived char[J].Chemical Engineering Journal,2010,156(2):321-327. |
| 32 | 汪怀建,涂翔,黄碧纯,等.生物炭应用于低温NH3-SCR脱硝催化剂的研究进展[J].江西农业大学学报,2021,43(2):445-458. |
| WANG Huaijian, TU Xiang, HUANG Bichun, et al.Review on application of biochar to denitrification catalysts for low-temperature NH3-SCR[J].Acta Agriculturae Universitatis Jiangxiensis,2021,43(2):445-458. | |
| 33 |
IWAMOTO M, FURUKAWA H, MINE Y, et al.Copper(Ⅱ) ion-exchanged ZSM-5 zeolites as highly active catalysts for direct and continuous decomposition of nitrogen monoxide[J].Journal of the Chemical Society,Chemical Communications,1986(16).Doi:10.1039/C39860001272.
doi: 10.1039/C39860001272 |
| 34 | PEREIRA M V L, NICOLLE A, BERTHOUT D.Hydrothermal aging effects on Cu-zeolite NH3-SCR catalyst[J].Catalysis Today,2015,258: 424-431. |
| 35 | SHWAN S, JANSSON J, OLSSON L, et al.Deactivation mechanisms of iron-exchanged zeolites for NH3-SCR applications[J].Catalysis Today,2015,258: 432-440. |
| 36 | 章凌,段宏昌,谭争国,等.用于柴油车尾气消除反应(NH3-SCR)的八元环沸石分子筛研究进展[J].高等学校化学学报,2020,41(1):19-27. |
| ZHANG Ling, DUAN Hongchang, TAN Zhengguo, et al.Recent advances in the preparation of 8MR zeolites for the selective catalytic reduction of NO x (NH3-SCR) in diesel engines[J].Chemical Journal of Chinese Universities,2020,41(1):19-27. | |
| 37 | 王彬宇,李莉,李菁,等.用工业固体废料合成沸石分子筛的研究进展[J].高等学校化学学报,2021,42(1):40-59. |
| WANG Binyu, LI Li, LI Jing, et al.Recent progresses on the synthesis of zeolites from the industrial solid wastes[J].Chemical Journal of Chinese Universities,2021,42(1):40-59. | |
| 38 | GELVES J F, DORKIS L, MÁRQUEZ M A, et al.Activity of an iron colombian natural zeolite as potential geo-catalyst for NH3-SCR of NO x [J].Catalysis Today,2019,320: 112-122. |
| 39 | FENG Xi, LIN Qingjin, CAO Yi, et al.Neodymium promotion on the low-temperature hydrothermal stability of a Cu/SAPO-34 NH3-SCR monolith catalyst[J].Journal of the Taiwan Institute of Chemical Engineers,2017,80: 805-812. |
| 40 |
LISI L, CIMINO S.Poisoning of SCR catalysts by alkali and alkaline earth metals[J].Catalysts,2020,10(12).Doi:10.3390/catal10121475.
doi: 10.3390/catal10121475 |
| 41 | LIU Chang, SHI Jianwen, GAO Chen, et al.Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NO x with NH3:A review[J].Applied Catalysis A:General,2016,522: 54-69. |
| 42 | SHEN Boxiong, LIU Ting.Deactivation of MnO x -CeO x /ACF catalysts for low-temperature NH3-SCR in the presence of SO2 [J].Acta Physico-Chimica Sinica,2010,26(11):3009-3016. |
| 43 | WANG Chizhong, YANG Shijian, CHANG Huazhen, et al.Dispersion of tungsten oxide on SCR performance of V2O5WO3/TiO2:Acidity,surface species and catalytic activity[J].Chemical Engineering Journal,2013,225: 520-527. |
| 44 | KIJLSTRA W S, BRANDS D S, POELS E K, et al.Kinetics of the selective catalytic reduction of NO with NH3 over MnO x /Al2O3 catalysts at low temperature[J].Catalysis Today,1999,50(1):133-140. |
| 45 | ZHAO Ziwei, LI Erwei, QIN Yu, et al.Density functional theory (DFT) studies of vanadium-titanium based selective catalytic reduction(SCR) catalysts[J].Journal of Environmental Sciences (China),2020,90: 119-137. |
| 46 | 郭梓阳,霍旺晨,张育新,等.锰基低温NH3-SCR脱硝催化剂的研究概述[J].材料导报,2021,35(13):13085-13099. |
| GUO Ziyang, HUO Wangchen, ZHANG Yuxin, et al.A review of Mn-based low temperature NH3-SCR denitration catalyst[J].Materials Reports,2021,35(13):13085-13099. | |
| 47 |
JIANG Ye, LAI Chengzhen, LI Qingyi, et al.The poisoning effect of KCl and K2O on CeO2-TiO2 catalyst for selective catalytic reduction of NO with NH3 [J].Fuel,2020,280.Doi:10.1016/j.fuel.2020.118638.
doi: 10.1016/j.fuel.2020.118638 |
| 48 |
JIANG Ye, LIU Tianyu, LAI Chengzhen, et al.Deactivation of CeO2-TiO2 catalyst by K2SO4 for NH3-SCR:An experimental and DFT study[J].Applied Surface Science,2021,547.Doi:10.1016/j.apsusc.2021.149196.
doi: 10.1016/j.apsusc.2021.149196 |
| 49 |
TANG Xiaolong, SHI Yiran, GAO Fengyu, et al.Promotional role of Mo on Ce0.3FeO x catalyst towards enhanced NH3-SCR catalytic performance and SO2 resistance[J].Chemical Engineering Journal,2020,398.Doi:10.1016/j.cej.2020.125619.
doi: 10.1016/j.cej.2020.125619 |
| 50 | PHULE A D, CHOI J H, KIM J H.High performance of catalytic sheet filters of V2O5-WO3/TiO2 for NO x reduction[J].Environmental Science and Pollution Research International,2021,28(26):34009-34016. |
| 51 | ZHANG Xianlong, LV Shuangshuang, ZHANG Xincheng, et al.Improvement of the activity and SO2 tolerance of Sb-modified Mn/PG catalysts for NH3-SCR at a low temperature[J].Journal of Environmental Sciences,2021,101: 1-15. |
| 52 |
XIONG Shangchao, PENG Yue, WANG Dong, et al.The role of the Cu dopant on a Mn3O4 spinel SCR catalyst:Improvement of low-temperature activity and sulfur resistance[J].Chemical Engineering Journal,2020,387 .Doi:10.1016/j.cej.2020.124090.
doi: 10.1016/j.cej.2020.124090 |
| 53 | 李锋,金保升,刘涛,范红梅.以工业级TiO2为载体负载不同助催化剂V2O5基脱氮催化剂的性能[J].环境化学,2005,24(4):374-378. |
| LI Feng, JIN Baosheng, LIU Tao, et al.Study of deNO x behavior of V2O5-based catalyst with different catalyst promoters supported on commercial anatase TiO2 [J].Environmental Chemistry,2005,24(4):374-378. | |
| 54 | 朱崇兵,金保升,李锋,等.V2O5-WO3/TiO2基催化剂SCR法脱硝试验研究[J].华东电力,2007,35(6):18-22. |
| ZHU Chongbing, JIN Baosheng, LI Feng, et al.Experiment study on SCR DeNO x behavior of V2O5-WO3/TiO2 catalysts[J].East China Electric Power,2007,35(6):18-22. |
| [1] | WU Bingdang, PAN Yinglu, ZHAO Qinzheng, WU Wei, XU Xiaoyi, HUANG Tianyin. Preparation of novel lanthanum coagulants and their deep phosphorus removal properties in low temperature and turbidity water [J]. Inorganic Chemicals Industry, 2024, 56(2): 127-135. |
| [2] | YAN Fangning,GUO Jinchun,HUANG Xueli,ZHOU Tingting,WANG Xueying,LUO Qinglong,ZOU Xuejing. Study on phase equilibrium of quinary system of Li+,Na+,Mg2+//SO42-,Cl--H2O at 258.15 K [J]. Inorganic Chemicals Industry, 2023, 55(2): 61-66. |
| [3] | XU Jingyao,ZHOU Xiaoli,SUN Jinghui,CAO Alin,QING Peilin. Study on preparation of flake α-alumina based on mixed molten salt at low temperature [J]. Inorganic Chemicals Industry, 2023, 55(2): 73-78. |
| [4] | YANG Tinglong,WANG Fuzhong,LIU Fei. Study on sulfur poisoning of zirconium-based bimetallic oxides catalyst [J]. Inorganic Chemicals Industry, 2023, 55(1): 151-158. |
| [5] | ZHANG Ji,NING Yansheng,GUO Xifeng,ZHU Jianping,WANG Kuo. Preparation of potassium water glass using manganese?containing silica gel at low temperature [J]. Inorganic Chemicals Industry, 2022, 54(5): 126-130. |
| [6] | DU Peining,LUO Shizhong,Tan Renjun,JING Fangli. Study on denitration performance of Y doped Fe2O3 catalyst [J]. Inorganic Chemicals Industry, 2022, 54(11): 137-142. |
| [7] | YU Wohui,ZHANG Pan,ZHAO Yiming,ZHA Zhongfa,WU Ruilong,QI Liqiang. Research progress on low-temperature denitration catalysts [J]. Inorganic Chemicals Industry, 2022, 54(10): 57-68. |
| [8] | Huang Li,Wang Hu,Zong Yuhao,Chang Zhengfeng,Liu Yang,Li Jinke,Han Pei. Study on deactivation effect of chromium on industrial V-Mo/Ti De-NOx catalyst [J]. Inorganic Chemicals Industry, 2021, 53(8): 112-116. |
| [9] | Liu Xingyu,Jia Yuanyuan,Wu Shufeng,Tang Zhonghua,Zhang Peng,Liu Junqiang,Pu Xin. Research progress of Ce-based SCR denitration catalysts [J]. Inorganic Chemicals Industry, 2021, 53(8): 15-20. |
| [10] | Su Shaolong,Qu Xiaolong,Zhong Dule,Sun Yanmin,Nan Jun,Li Shipeng. Study on preparation and denitration performance of Cu-Ce/ZSM-5 zeolite catalyst [J]. Inorganic Chemicals Industry, 2021, 53(6): 190-193. |
| [11] | Zhou Anhui,Qiu Zhaofu,Yang Ji,Yan Ruiqi,Bian Xiaotong. Analysis of pollutants and environmental risk in spent SCR catalyst [J]. Inorganic Chemicals Industry, 2021, 53(2): 11-16. |
| [12] | WEN Zhigang,RUAN Daitan,ZHU Jiachan,LUO Qiaofeng,ZHANG Xiaoyang. Preparation of ceramic glaze at low temperature [J]. Inorganic Chemicals Industry, 2021, 53(12): 109-112. |
| [13] | SUN Chunhui,CHEN Yongsheng,LIU Wei,XU Yan,ZHANG Jingcheng,YU Ruixiang. Study on new preparation process and performance of V2O5 /(TiO2-SiO2) denitrification catalyst [J]. Inorganic Chemicals Industry, 2021, 53(12): 146-149. |
| [14] | Feng Qing,Zeng Xianjun,Zhang Lijie,Li He,Meng Guangying,Sun Yanmin. Summary of transition metal manganese-based denitration catalyst for low temperature NH3-SCR [J]. Inorganic Chemicals Industry, 2021, 53(1): 7-13. |
| [15] | Gan Yonghai,Wu Bingdang,Li Haojie,Li Jingbiao,Li Runsheng,Zhang Shujuan. Preparation and application evaluation of a novel titanium coagulant [J]. Inorganic Chemicals Industry, 2020, 52(9): 1-5. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||