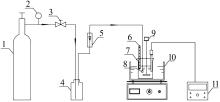
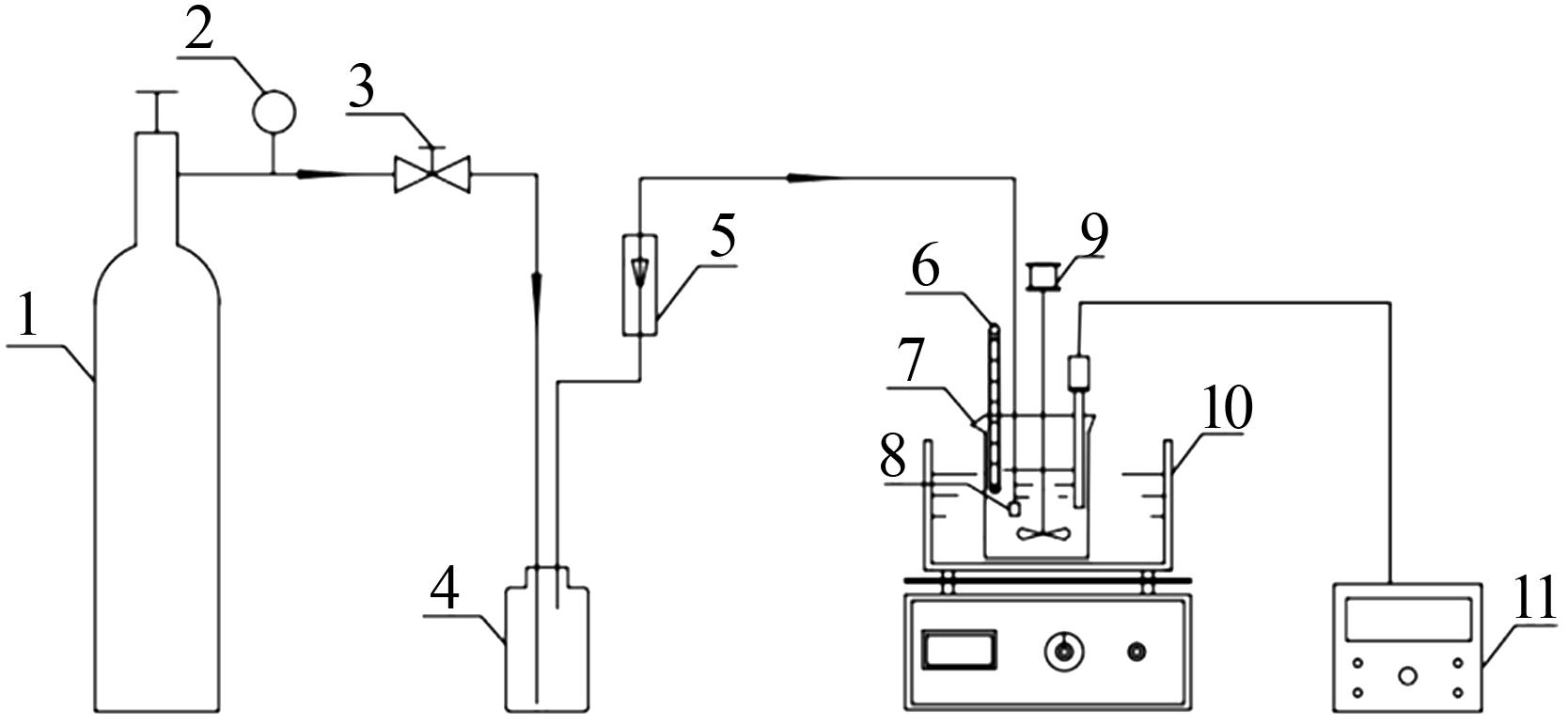
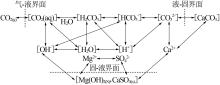
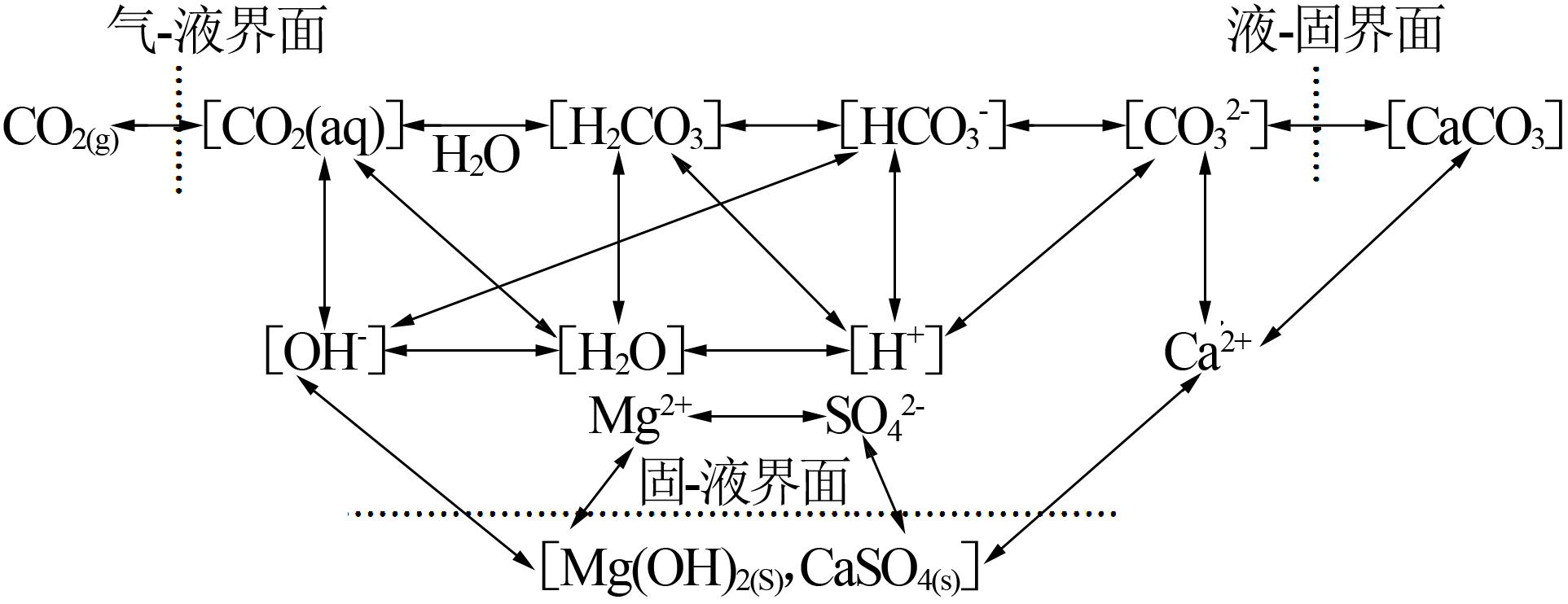
Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (6): 120-124.doi: 10.19964/j.issn.1006-4990.2021-0580
• Environment·Health·Safety • Previous Articles Next Articles
Study on macro?kinetics of carbonization reaction of ammonia alkali salt sludge
ZHANG Shaokang( ),ZHAO Hua,LIU Runjing(
),ZHAO Hua,LIU Runjing( )
)
- College of Chemical and Pharmaceutical Engineering,Hebei University of Science and Technology,Shijiazhuang 050018,China
-
Received:2021-09-24Online:2022-06-10Published:2022-06-22 -
Contact:LIU Runjing E-mail:zsk19952021@163.com;liurj@hebust.edu.cn
CLC Number:
Cite this article
ZHANG Shaokang,ZHAO Hua,LIU Runjing. Study on macro?kinetics of carbonization reaction of ammonia alkali salt sludge[J]. Inorganic Chemicals Industry, 2022, 54(6): 120-124.
share this article
| 1 | 刘润静,刘兴,罗慧慧,等.氯化铵浸取法回收盐泥中的镁[J].化工环保,2019,39(5):552-556. |
| LIU Runjing, LIU Xing, LUO Huihui,et al.Recovery of magnesium from salt sludge by leaching with ammonium chloride[J].Environmental Protection of Chemical Industry,2019,39(5):552-556. | |
| 2 | 王锐清.氨碱法纯碱生产废渣的开发利用进展[J].纯碱工业,2005(6):16-19. |
| WANG Ruiqing.Progress on exploitation and utilization of waste residue in ammonia soda industry[J].Soda Industry,2005(6):16-19. | |
| 3 | 张润泽.氨碱法纯碱生产中废液废渣的治理和综合利用[J].化工管理,2015(8):225. |
| ZHANG Runze.The comprehensive utilization and harnessing of waste liquid and waste residuce in producing process of sodium carbonate[J].Chemical Enterprise Management,2015(8):225. | |
| 4 | 王晓娜,张云净,王亚飞,等.氨碱法纯碱厂废渣综合利用[J].化学工程师,2014,28(2):32-34. |
| WANG Xiaona, ZHANG Yunjing, WANG Yafei,et al.Comprehensive utilization for alkaline slag in the soda production by ammonia⁃soda process[J].Chemical Engineer,2014,28(2):32-34. | |
| 5 | 朱春来,刘素芹.基于氨碱法纯碱生产中废液及碱渣的综合利用研究[J].化工管理,2018(14):144-145. |
| ZHU Chunlai, LIU Suqin.Study on comprehensive utilization of waste liquid and alkali residue in soda ash production based on ammonia⁃alkali method[J].Chemical Enterprise Management,2018(14):144-145. | |
| 6 | 崔耀星,陈留平,徐俊辉,等.盐泥资源综合利用的研究进展[J].氯碱工业,2018,54(4):33-36,40. |
| CUI Yaoxing, CHEN Liuping, XU Junhui,et al.Research progress on comprehensive utilization of salt mud resources[J].Chlor⁃Alkali Industry,2018,54(4):33-36,40. | |
| 7 | 颜鑫,吴健谊,卢云峰,等.氨碱厂碱渣充分综合利用新技术的研究[J].无机盐工业,2021,53(1):68-71. |
| YAN Xin, WU Jianyi, LU Yunfeng,et al.Study on new technology of comprehensive utilization of alkali residue in ammonia alkali plant[J].Inorganic Chemicals Industry,2021,53(1):68-71. | |
| 8 | 黎艳,王娟芳,吴彬,等.利用盐泥制取轻质氧化镁[J].化工环保,2013,33(2):144-148. |
| LI Yan, WANG Juanfang, WU Bin,et al.Preparation of light magnesium oxide from salt mud[J].Environmental Protection of Che⁃ | |
| Industry mical,2013,33(2):144-148. | |
| 9 | 孙维兵,侯梦迪.浅谈石灰纯碱法精制盐水[J].纯碱工业,2019(2):11-13. |
| SUN Weibing, HOU Mengdi.Refining brine technique by using lime⁃soda method[J].Soda Industry,2019(2):11-13. | |
| 10 | 云玉娥,刘艳娟,王秉钧.盐水精制过程中用硫酸钠除钙可行性试验分析[J].化学工程师,2016,30(6):79-80,72. |
| YUN Yue, LIU Yanjuan, WANG Bingjun.Experimentation of the brine purity for removing calcium by sodium sulfate[J].Chemical Engineer,2016,30(6):79-80,72. | |
| 11 | 刘润静,刘兴,赵华,等.一种盐泥生产轻质碳酸钙和七水硫酸镁的方法:中国,109574055B[P].2021-03-26. |
| LIU Runjing, LIU Xing, ZHAO Hua,et al.A method for producing light calcium carbonate and magnesium sulfate heptahydrate from salt mud:CN,109574055B[P].2021-03-26. | |
| 12 | 陈建峰,刘润静,沈志刚,等.超重力反应沉淀法制备碳酸钙的过程与形态控制[J].过程工程学报,2002,2(4):309-313. |
| CHEN Jianfeng, LIU Runjing, SHEN Zhigang,et al.High⁃gravity reactive precipitation process and morphology control for precipitated calcium carbonate[J].The Chinese Journal of Process Engineering,2002,2(4):309-313. | |
| 13 | 周相廷,翟学良,刘百年,等.Mg(OH)2的结晶性和粒度对碳酸化的影响:Ⅱ.影响Mg(OH)2浆液碳酸化的因素[J].无机盐工业,1996,28(5):13-15. |
| ZHOU Xiangting, ZHAI Xueliang, LIU Bainian,et al.Effect of Mg(OH)2 crystallinity and particle size Mg(OH)2 on the carbonationⅡ.Factors affecting the carbonation of the slurry[J].Inorganic Chemicals Industry,1996,28(5):13-15. | |
| 14 | YANG Weiguo, WANG Jinfu, JIN Yong.Gas⁃liquid mass transfer in a slurry bubble column reactor under high temperature and high pressure[J].Chinese Journal of Chemical Engineering,2001, |
| 9(3):253-257. | |
| 15 |
DU Yi, FU Changqing, GONG Bengen,et al.Real⁃time investigation of the CO2 mineral carbonation reaction rate through direct aqueous route using semi-dry desulfurization slag[J].Journal of CO2 Utilization,2021,51.Doi:10.1016/j.jcou.2021.101614 .
doi: 10.1016/j.jcou.2021.101614 |
| 16 | BHARADWAJ H K, LEE J Y, LI Xin,et al.Dissolution kinetics of magnesium hydroxide for CO2 separation from coal⁃fired power plants[J].Journal of Hazardous Materials,2013,250-251:292- 297. |
| 17 | ANTONY M P,JHA A, TATHAVADKAR V.Alkali roasting of Indian chromite ores:Thermodynamic and kinetic consideratio⁃ |
| ns[J].Mineral Processing and Extractive Metallurgy,2006,115(2):71-79. | |
| 18 | 伊元荣,韩敏芳.钢渣湿法捕获CO2反应机制研究[J].环境科学与技术,2013,36(6):159-163,190. |
| YI Yuanrong, HAN Minfang.Study on reaction mechanism of CO2 capture by wet steel slag[J].Environmental Science & Technology,2013,36(6):159-163,190. | |
| 19 | 王余莲,印万忠,张夏翔,等.三水碳酸镁法制备碱式碳酸镁过程研究[J].矿产保护与利用,2017(4):81-86. |
| WANG Yulian, YIN Wanzhong, ZHANG Xiaxiang,et al.Preparation of hydromagnesite using nesquehonite method[J].Conservation and Utilization of Mineral Resources,2017(4):81-86. | |
| 20 | 伊文涛,闫春燕,马培华.碳酸锂常压鼓泡碳化宏观反应动力学研究[J].化工矿物与加工,2009,38(3):1-4,27. |
| YI Wentao, YAN Chunyan, MA Peihua.Macro⁃kinetics study on bubbling carbonation of Li2CO3 slurries at atmospheric pressure[J].Industrial Minerals & Processing,2009,38(3):1-4,27. | |
| 21 | CATTANEO A, GUALTIERI A F, ARTIOLI G.Kinetic study of the dehydroxylation of chrysotile asbestos with temperature by in situ XRPD[J].Physics and Chemistry of Minerals,2003,30(3):177-183. |
| 22 | 朱炳辰.化学反应工程[M].北京:化学工业出版社,1993. |
| ZHU Bingchen.Chemical reaction engineering[M].Beijing:Che⁃ | |
| mical Industy Press,1993. | |
| 23 | XIE Pengfei, LI Liqing, HE Zhicheng,et al.Gas⁃liquid mass trans⁃ |
| fer of carbon dioxide capture by magnesium hydroxide slurry in a bubble column reactor[J].Journal of Central South University,2019,26(6):1592-1606. | |
| 24 | ASHRAF W, OLEK J.Carbonation activated binders from pure calcium silicates:Reaction kinetics and performance controlling factors[J].Cement and Concrete Composites,2018,93:85-98. |
| 25 | 丁珂,孙晓君,李会杰,等.氧化镁蒸氨反应的动力学和机理研究[J].无机盐工业,2019,51(11):31-35. |
| DING Ke, SUN Xiaojun, LI Huijie,et al.Study on kinetics and mechanism of ammonia evaporation reaction of magnesium oxi⁃ | |
| de[J].Inorganic Chemicals Industry,2019,51(11):31-35. |
| [1] | ZHANG Yaqi, CHEN Xiping, SUN Ningning. Study on preparation process of cryolite from anode carbon slag by catalytic decarburization [J]. Inorganic Chemicals Industry, 2024, 56(3): 91-97. |
| [2] | XIANG Mengqi, MENG Hua, WANG Ye, MENG Xianzhang, BAI Yuhang, WANG Yujunyao, ZHANG Yidan. Study on kinetic of iron leaching from titanium gypsum and its cyclic acid leaching process [J]. Inorganic Chemicals Industry, 2024, 56(1): 114-120. |
| [3] | ZHOU Zhaoan, LI Jun, LIU Xiaowen, ZHOU Aiqing, MAO Anzhang. Study on carbonization and purification process of high COD industrial waste salt [J]. Inorganic Chemicals Industry, 2023, 55(9): 100-105. |
| [4] | CHEN Yanmeng, MO Huiling, ZHONG Jiamei, YANG Pengfei, LAN Junfeng. Study on preparation process of porous calcium carbonate by carbonization [J]. Inorganic Chemicals Industry, 2023, 55(8): 102-108. |
| [5] | REN Teng, LI Shengdong, WANG Dexi, CHU Fuzhou, SHAO Lixin. Numerical simulation analysis of influence of jet position on mixing effect of carbonization reactor [J]. Inorganic Chemicals Industry, 2023, 55(7): 109-114. |
| [6] | QU Xiaoyuan, ZHENG Qiang, FAN Yuanyang, LIU Haili, DENG Xiaoyang, LI Xue. Study on preparation of large cube calcium carbonate by secondary carbonization of dolomite [J]. Inorganic Chemicals Industry, 2023, 55(7): 58-64. |
| [7] | TIAN Peng, ZHOU Ruohui, XU Qianjin, LIU Kunji, PANG Hongchang, NING Guiling. Synthesis and dehydration dynamics of boehmite microcrystalline with different particle sizes [J]. Inorganic Chemicals Industry, 2023, 55(11): 27-36. |
| [8] | ZHANG Shaokang,ZHAO Hua,LIU Runjing. Study on macro?kinetics of carbonization reaction of ammonia alkali salt sludge [J]. Inorganic Chemicals Industry, 2022, 54(6): 120-124. |
| [9] | QIAO Kun,LÜ Zening,YANG Lijun,DU Xiaoze. Research progress on effect of inorganic additives on regeneration process of carbon capture by ammonia [J]. Inorganic Chemicals Industry, 2022, 54(10): 79-86. |
| [10] | Yang Wenzhen,Xiong Ping,Sun Xiuyun,Han Weiqing. Study on combustion characteristics of waste pharmaceutical Na2SO4 and impurity removal by low-temperature carbonization [J]. Inorganic Chemicals Industry, 2021, 53(9): 76-82. |
| [11] | Zhao Li,Zhuo Minquan,Gong Fuzhong,Wang Jun,Ruan Heng,Li Kaicheng,Li Yanlin. Synthesis of vaterite CaCO3 microspheres by carbonization method and its formation mechanism [J]. Inorganic Chemicals Industry, 2021, 53(3): 38-43. |
| [12] | XIE Juan,LIU Songlin,TAO Shaocheng,HU Houmei,LIU Xu. Study on preparation of magnesium carbonate hydroxide by carbonization method from high-Mg phosphate tailings [J]. Inorganic Chemicals Industry, 2021, 53(12): 135-139. |
| [13] | XU Daying,GENG Wenjuan,QIAN Dequan,XUE Rui. Influence of several carbohydrates on crystallization of nano calcium carbonate [J]. Inorganic Chemicals Industry, 2021, 53(11): 77-80. |
| [14] | Yang Xiaohong,Xue Xishi,Zhang Lulu,Chang Jun. Kinetics study of calcium leaching from electrolytic manganese residue by hydrochloric acid [J]. Inorganic Chemicals Industry, 2021, 53(1): 82-86. |
| [15] | Zhang Yan,Zhao Zhenzhong,Ma Zhaohui,Zhao Yancai,Chen Zhiyu,Ma Zhengqiang. Optimization of process for preparation of battery grade lithium carbonate by carbonization [J]. Inorganic Chemicals Industry, 2020, 52(3): 68-71. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||