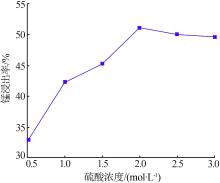
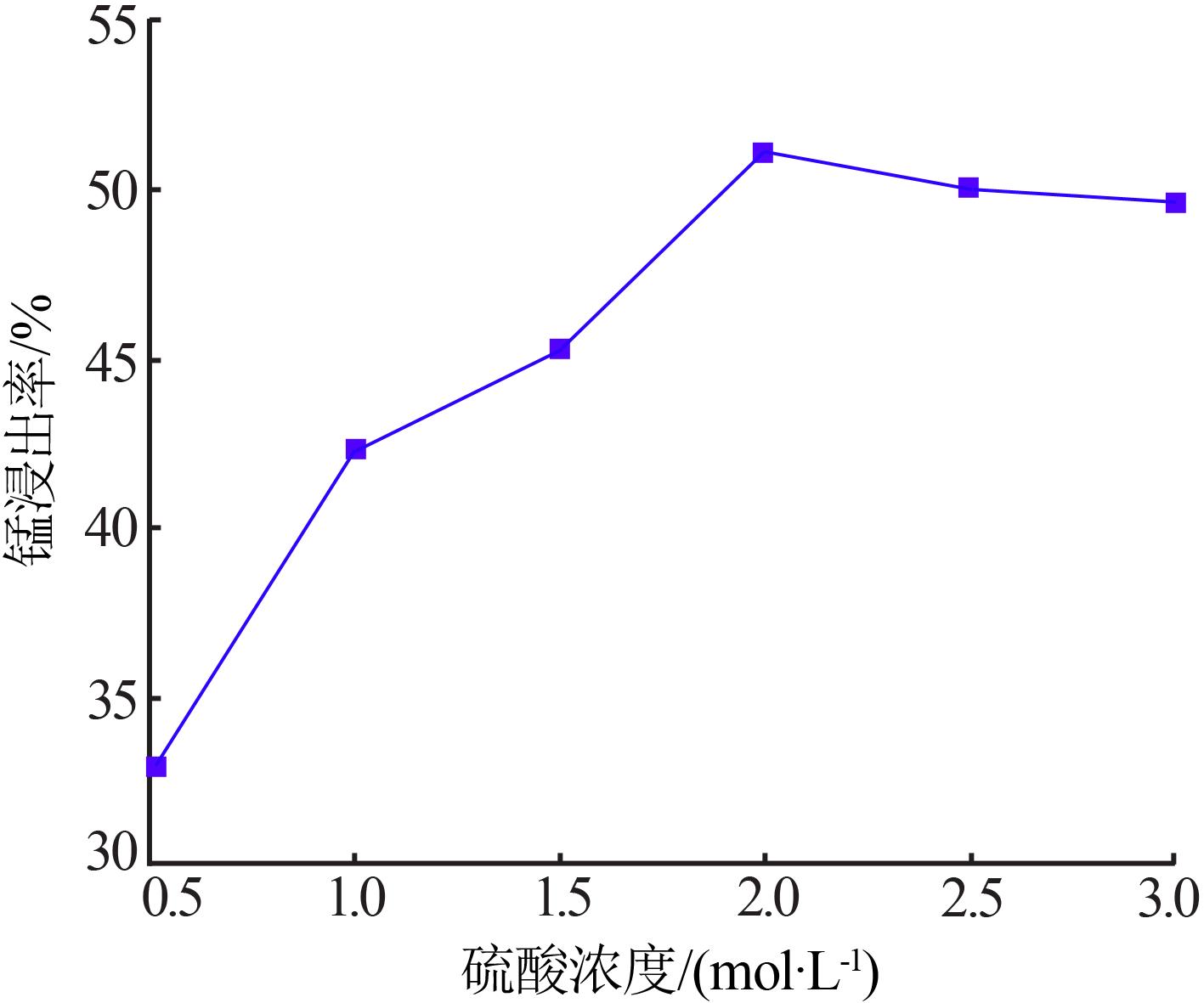
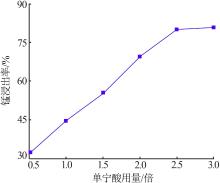
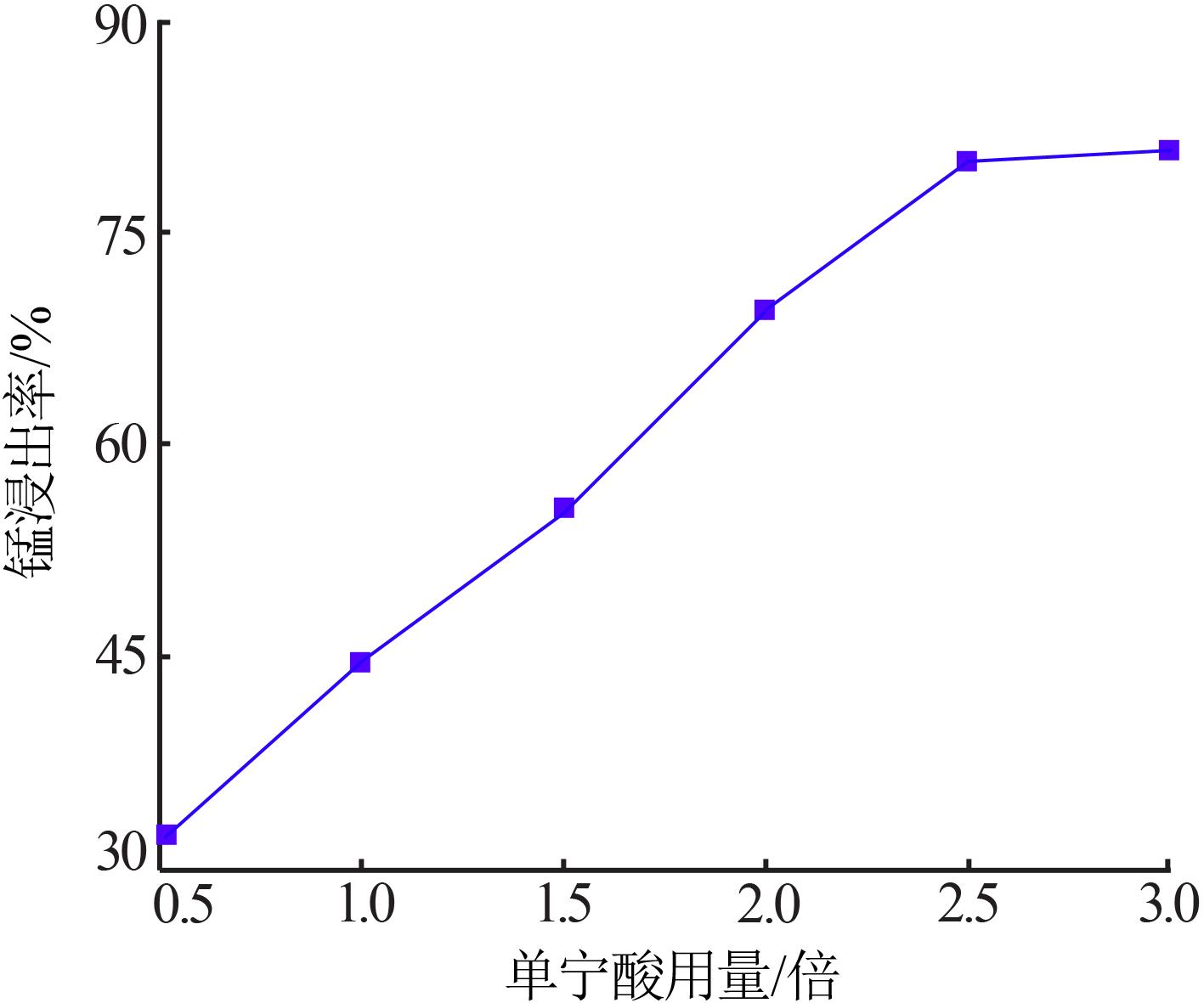
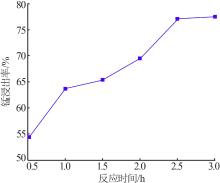
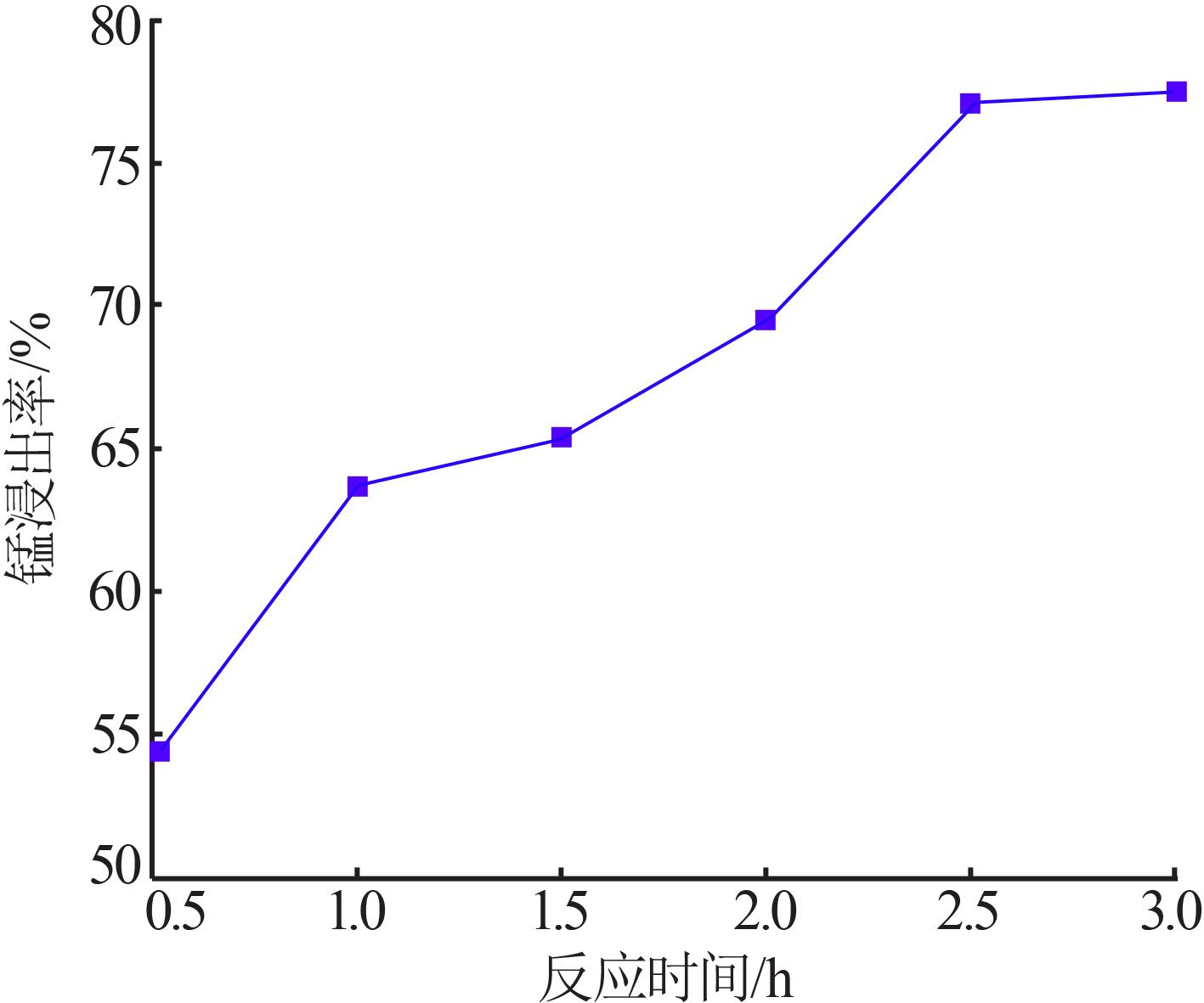
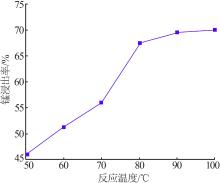
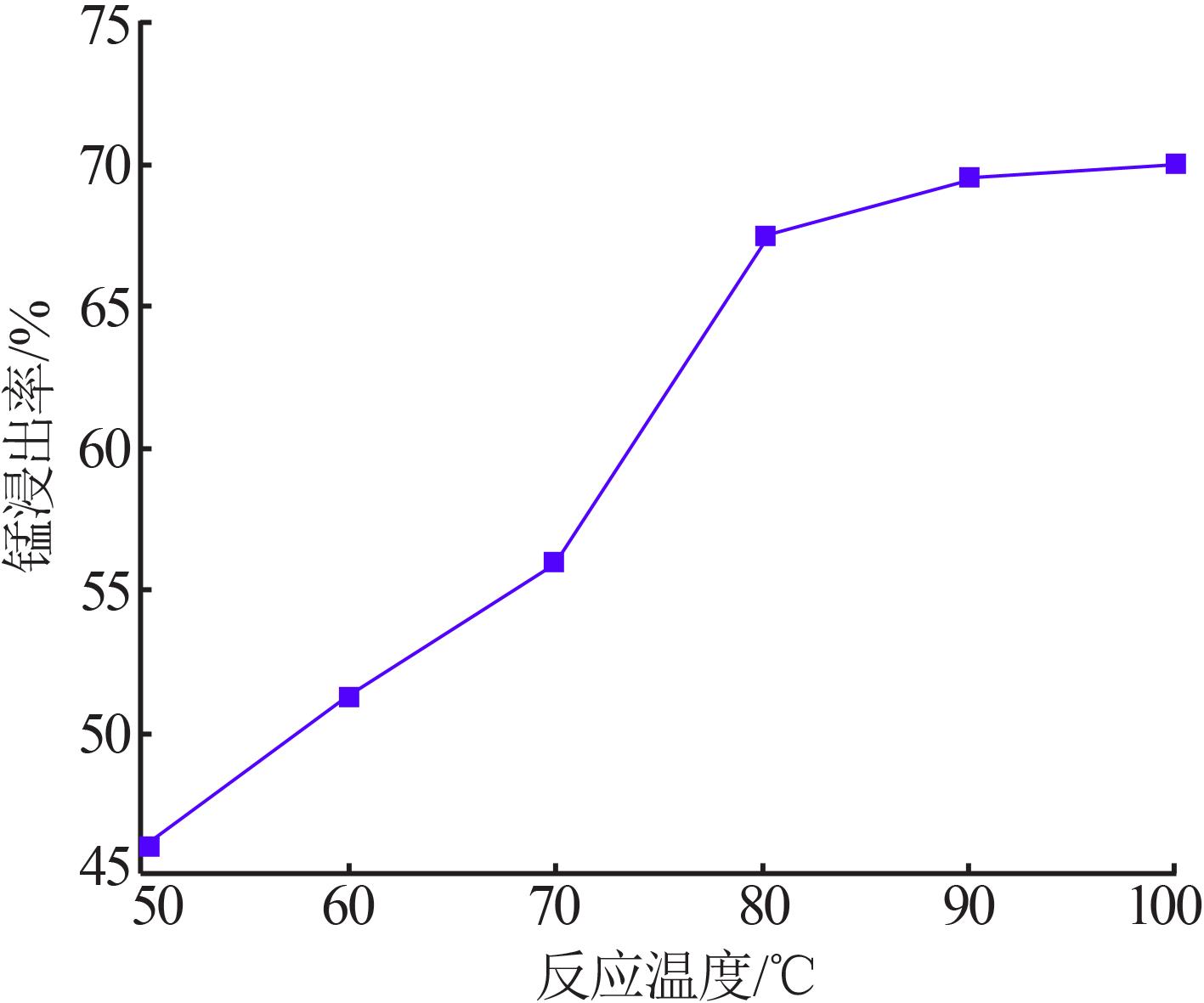
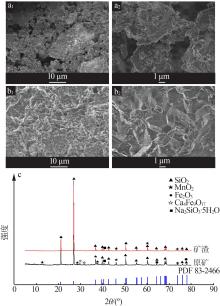
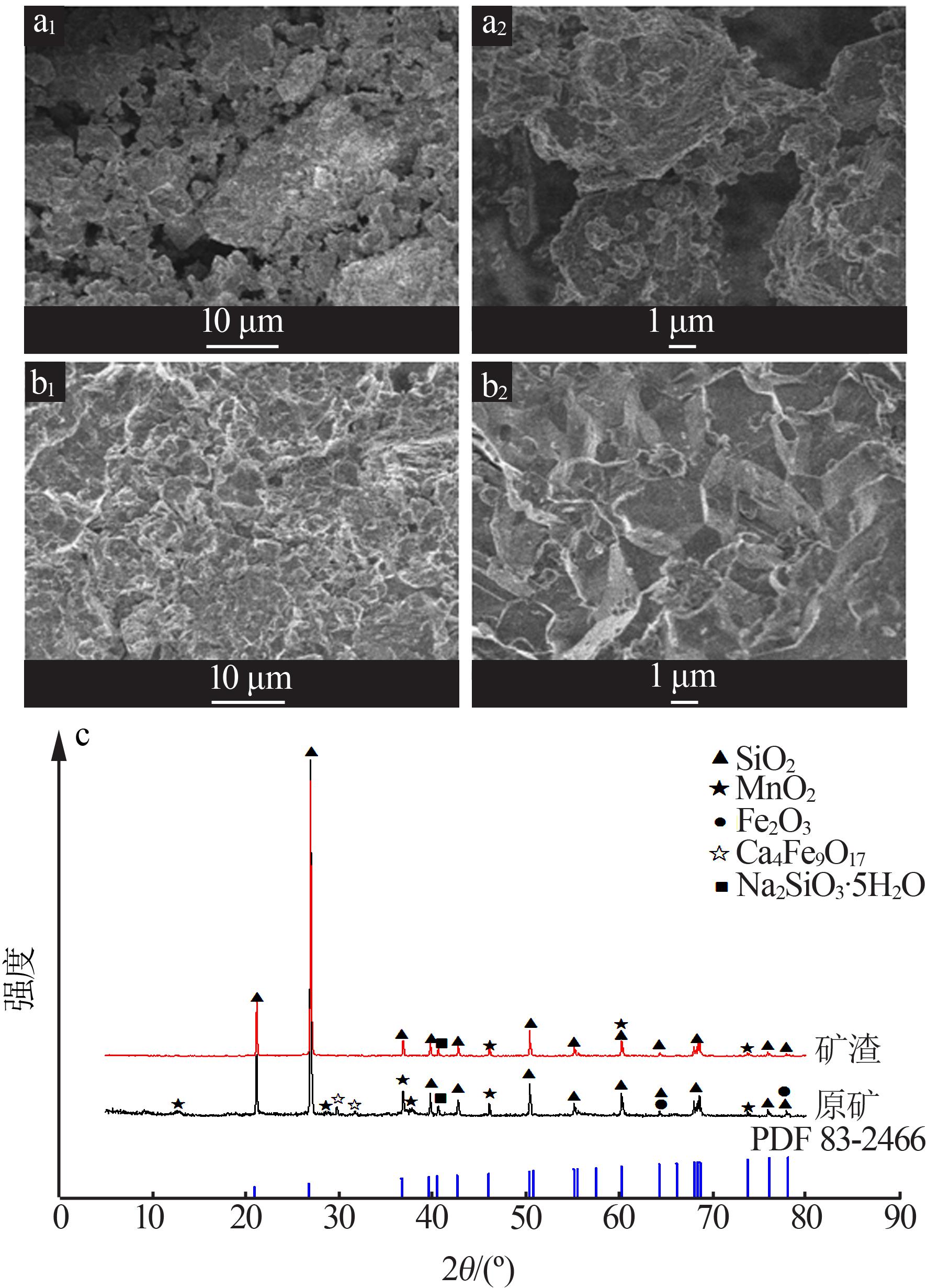
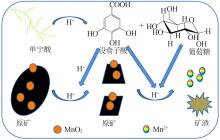
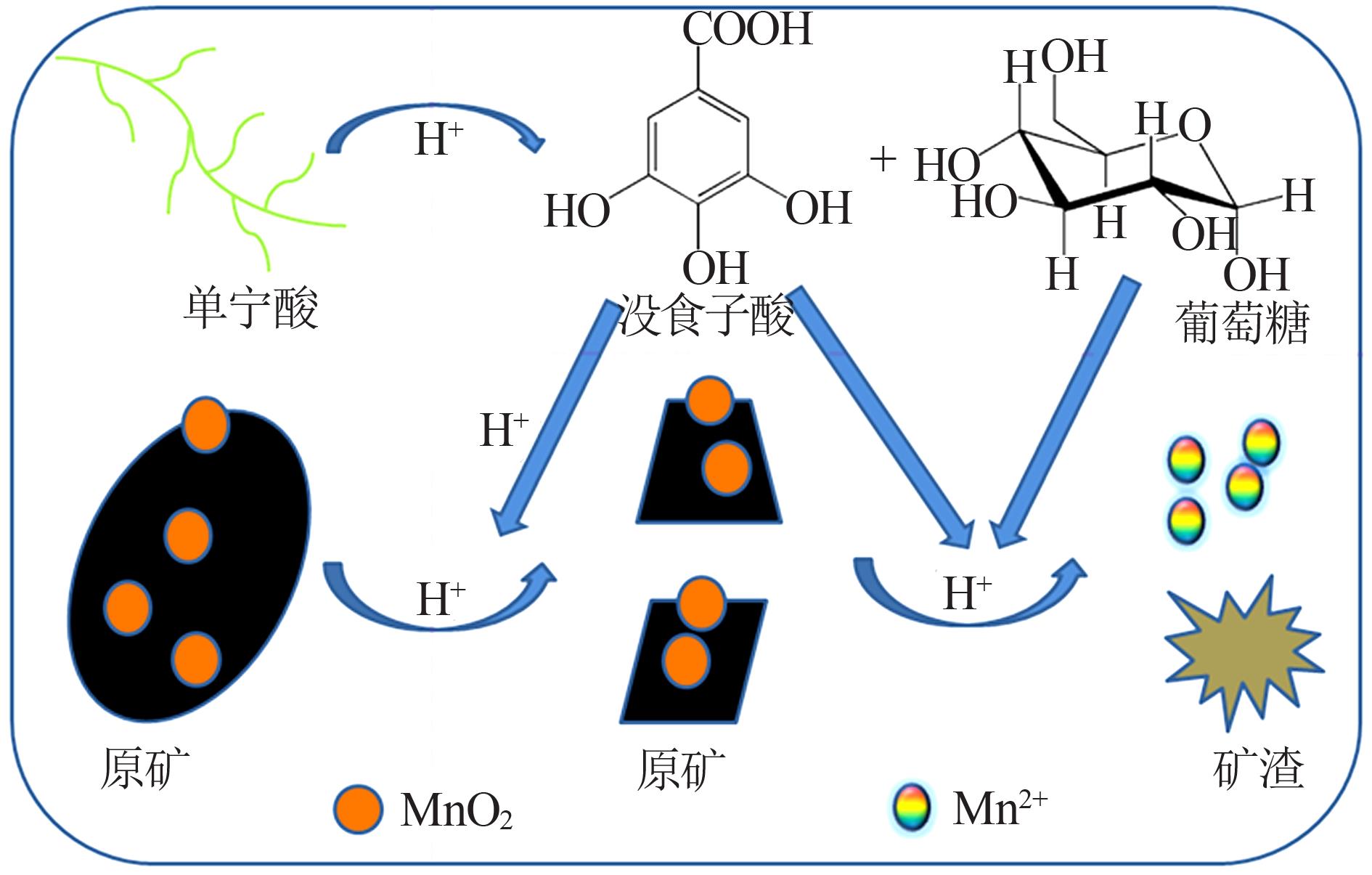
Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (6): 84-89.doi: 10.19964/j.issn.1006-4990.2021-0526
• Research & Development • Previous Articles Next Articles
Study on leaching of manganese from manganese dioxide ore with tannic acid?sulfuric acid system
- Guilin University of Technology at Nanning,Nanning 530001,China
-
Received:2021-09-27Online:2022-06-10Published:2022-06-22
CLC Number:
Cite this article
LU Youzhi,SU Limin,ZHAO Yi. Study on leaching of manganese from manganese dioxide ore with tannic acid?sulfuric acid system[J]. Inorganic Chemicals Industry, 2022, 54(6): 84-89.
share this article
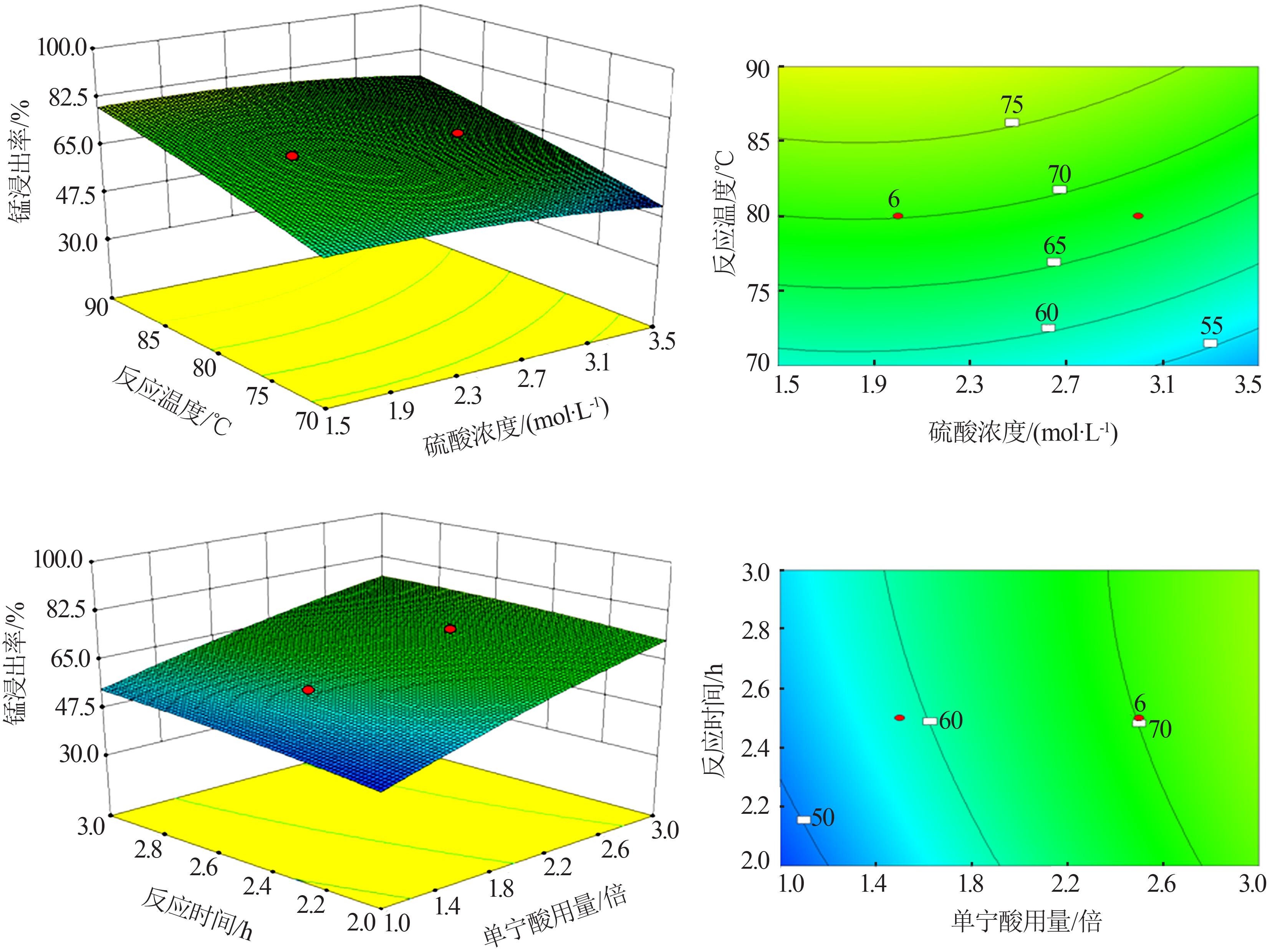
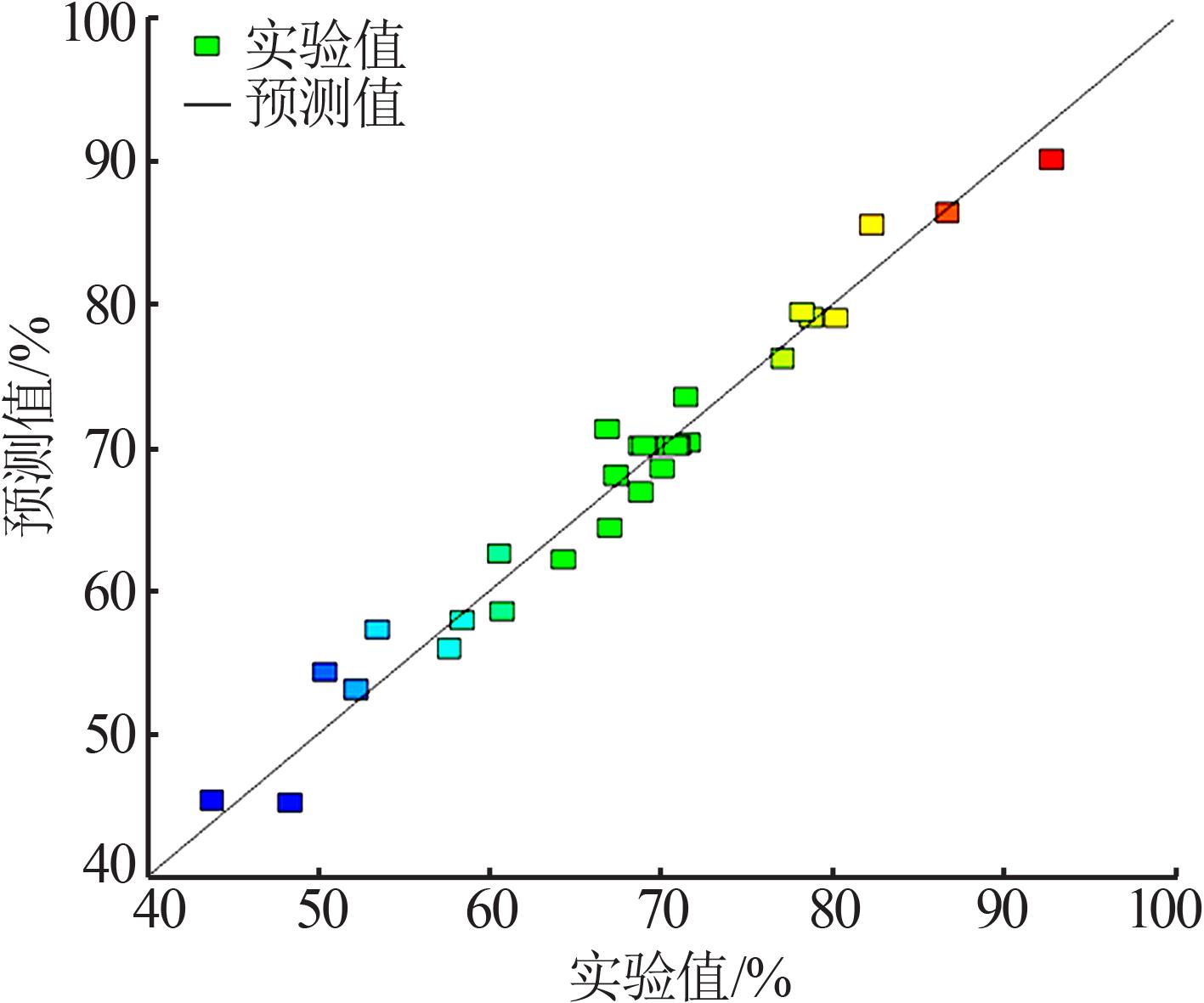
Table 1
Experimental design and results"
| 序号 | A 硫酸浓度/ (mol?L-1) | B 单宁酸 用量/倍 | C 浸出时间/ h | D 反应温度/ ℃ | Y 浸出率/ % |
|---|---|---|---|---|---|
| 1 | 1.5 | 2.0 | 2.0 | 90 | 67.101 8 |
| 2 | 2.5 | 3.0 | 2.0 | 90 | 78.851 1 |
| 3 | 1.5 | 3.0 | 3.0 | 70 | 67.415 1 |
| 4 | 2.0 | 2.5 | 2.5 | 80 | 69.086 2 |
| 5 | 2.0 | 2.5 | 3.5 | 80 | 71.697 1 |
| 6 | 2.0 | 1.5 | 2.5 | 80 | 60.835 5 |
| 7 | 2.5 | 2.0 | 2.0 | 80 | 71.540 4 |
| 8 | 1.0 | 2.5 | 2.5 | 80 | 70.130 5 |
| 9 | 2.0 | 2.5 | 2.5 | 80 | 68.877 3 |
| 10 | 2.0 | 2.5 | 2.5 | 80 | 70.913 8 |
| 11 | 2.5 | 2.0 | 3.0 | 90 | 77.180 2 |
| 12 | 2.0 | 2.5 | 2.5 | 100 | 86.788 5 |
| 13 | 2.0 | 2.5 | 2.5 | 80 | 71.122 7 |
| 14 | 1.5 | 3.0 | 3.0 | 90 | 92.890 2 |
| 15 | 2.5 | 3.0 | 3.0 | 70 | 53.524 8 |
| 16 | 2.0 | 3.5 | 2.5 | 80 | 80.313 3 |
| 17 | 2.0 | 2.5 | 2.5 | 80 | 70.235 0 |
| 18 | 2.0 | 2.5 | 2.5 | 80 | 70.548 3 |
| 19 | 2.5 | 3.0 | 3.0 | 90 | 78.329 0 |
| 20 | 1.5 | 2.0 | 3.0 | 70 | 52.271 5 |
| 21 | 1.5 | 2.0 | 3.0 | 90 | 66.945 2 |
| 22 | 2.0 | 2.5 | 2.5 | 60 | 48.407 3 |
| 23 | 2.0 | 2.5 | 1.5 | 80 | 64.386 4 |
| 24 | 2.5 | 2.0 | 2.0 | 70 | 50.443 9 |
| 25 | 1.5 | 3.0 | 2.0 | 70 | 60.626 6 |
| 26 | 1.5 | 3.0 | 2.0 | 90 | 82.402 1 |
| 27 | 3.0 | 2.5 | 2.5 | 80 | 68.877 3 |
| 28 | 1.5 | 2.0 | 2.0 | 70 | 43.864 2 |
| 29 | 2.5 | 2.0 | 3.0 | 70 | 58.485 6 |
| 30 | 2.5 | 3.0 | 2.0 | 70 | 57.702 3 |
Table 1
Table 2
Variance analysis results"
| 项目 | 均方和 | 自由度 | 均方 | F | P |
|---|---|---|---|---|---|
| 模型 | 3 626.16 | 14 | 259.01 | 31.18 | <0.000 1 |
| A | 4.14 | 1 | 4.14 | 0.499 0 | 0.490 8 |
| B | 629.06 | 1 | 629.06 | 75.73 | <0.000 1 |
| C | 100.61 | 1 | 100.61 | 12.11 | 0.003 4 |
| D | 2 555.98 | 1 | 2 555.98 | 307.72 | <0.000 1 |
| AB | 243.38 | 1 | 243.38 | 29.30 | <0.000 1 |
| AC | 17.13 | 1 | 17.13 | 2.06 | 0.171 6 |
| AD | 0.020 6 | 1 | 0.020 6 | 0.002 5 | 0.960 9 |
| BC | 5.46 | 1 | 5.46 | 0.6574 | 0.430 2 |
| BD | 15.03 | 1 | 15.03 | 1.81 | 0.198 5 |
| CD | 0.811 4 | 1 | 0.811 4 | 0.097 7 | 0.758 9 |
| A2 | 10.13 | 1 | 10.13 | 1.22 | 0.286 9 |
| B2 | 3.17 | 1 | 3.17 | 0.381 4 | 0.546 0 |
| C2 | 25.97 | 1 | 25.97 | 3.13 | 0.097 3 |
| D2 | 32.24 | 1 | 32.24 | 3.88 | 0.067 6 |
Table 2
| 1 | 刘其静.单宁酸鞣制对牛心包材料的改性研究[D].武汉:华中农业大学,2019. |
| LIU Qijing.Study on modification of bovine pericardium material with tannic acid[D].Wuhan:Huazhong Agricultural University,2019. | |
| 2 | 孔维悦,汪国庆,万逸,等.单宁酸/醇溶性壳聚糖复合材料的制备及其生物性能[J].腐蚀与防护,2021,42(6):38-43,67. |
| KONG Weiyue, WANG Guoqing, WAN Yi,et al.Preparation and biological properties of tannic acid/alcohol-soluble chitosan compound[J].Corrosion & Protection,2021,42(6):38-43,67. | |
| 3 | 严浩钊,周新华,周红军.单宁酸合成功能材料的研究进展[J].仲恺农业工程学院学报,2021,34(2):64-71. |
| YAN Haozhao, ZHOU Xinhua, ZHOU Hongjun.Research progress of tannic acid synthetic functional materials[J].Journal of Zhongkai University of Agriculture and Engineering,2021,34(2):64-71 | |
| 4 | 邵延林,陈国木,潘明熙,等.聚合氯化铝去除浸出液中单宁酸的影响因素及机理[J].有色金属工程,2021,11(6):49-58. |
| SHAO Yanlin, CHEN Guomu, PAN Mingxi,et al.Influencing factors and mechanism of polyaluminum chloride to removing tannic acid in leaching solution[J].Nonferrous Metals Engineering,2021,11(6):49-58. | |
| 5 | 卢友志,黄润均,王军正.低品位软锰矿无机还原浸出研究进展[J].湿法冶金,2019,38(6):427-431. |
| LU Youzhi, HUANG Runjun, WANG Junzheng.Research progress on reductive leaching of low grade pyrolusite by inorganic substance[J].Hydrometallurgy of China,2019,38(6):427-431. | |
| 6 | SINHA M K, PURCELL W.Reducing agents in the leaching of manganese ores:A comprehensive review[J].Hydrometallurgy,2019,187:168-186. |
| 7 | 卢友志,卢国贤,明宪权,等.有机物还原氧化锰工艺研究进展[J].湿法冶金,2015,34(1):1-5. |
| LU Youzhi, LU Guoxian, MING Xianquan,et al.Research advances of reduction of manganese oxide ore by organic matter[J].Hydrometallurgy of China,2015,34(1):1-5. | |
| 8 | 张亚辉,栾和林,姚文,等.苯酚或苯胺还原浸出大洋锰结核的机理研究与验证[J].矿冶,1997,6(1):52-58. |
| ZHANG Yahui, LUAN Helin, YAO Wen,et al.Research and verification on reduction leaching mechanism of ocean manganese nodules with aniline or phenol[J].Mining and Metallurgy,1997,6(1):52-58. | |
| 9 | FURLANI G, PAGNANELLI F, TORO L.Reductive acid leaching of manganese dioxide with glucose:Identification of oxidation derivatives of glucose[J].Hydrometallurgy,2006,81(3/4):234-240. |
| 10 | 冯雅丽,康金星,李浩然,等.焦化废水还原浸出软锰矿动力学[J].中国有色金属学报,2017,27(5):1051-1060. |
| FENG Yali, KANG Jinxing, LI Haoran,et al.Leaching kinetics of pyrolusite ores using coking wastewater as reductant[J].The Chinese Journal of Nonferrous Metals,2017,27(5):1051-1060. | |
| 11 | 王雨红,雷作敏,屈欣轲,等.葡萄糖在氧化锰矿浸出过程中的分解动力学[J].中国有色金属学报,2016,26(6):1303-1310. |
| WANG Yuhong, LEI Zuomin, QU Xinke,et al.Oxidative breakdown kinetics of glucose in process of leaching mangaese oxide ore[J].The Chinese Journal of Nonferrous Metals,2016,26(6):1303-1310. | |
| 12 | 粟海锋,崔嵬,孙英云,等.废糖蜜-硫酸溶液中软锰矿的浸出动力学[J].过程工程学报,2010,10(3):542-547. |
| SU Haifeng, CUI Wei, SUN Yingyun,et al.Leaching kinetics of pyrolusite using cane molasses as reductant in sulfuric acid solution[J].The Chinese Journal of Process Engineering,2010,10(3):542-547. |
| [1] | ZHANG Conghua, YAN Wenbin, XIAO Jiajun, ZHAO Ke, PENG Shangquan, WEI Yuhong. Reductive leaching technology of manganese anode slag using tartaric acid as reducing agent optimized by RSM [J]. Inorganic Chemicals Industry, 2023, 55(9): 106-113. |
| [2] | WANG Yingnan, SHENG Linlin, HUANG Juan, HUANG Zhanbin. Study on adsorption performance of lead from water by coal-fired slag [J]. Inorganic Chemicals Industry, 2023, 55(8): 109-115. |
| [3] | CUI Gengyin, XIE Lang, LU Yuexian, KONG Dewen, WANG Lingling. Optimization of mechanical properties of basalt fiber reinforced phosphogypsum-based composites based on RSM [J]. Inorganic Chemicals Industry, 2023, 55(8): 116-123. |
| [4] | WU Yulin, WU Junhu, YANG Xiushan, XU Dehua, ZHANG Zhiye. Study on optimization of decalcification process of calcium and magnesium nitrate solution by response surface method [J]. Inorganic Chemicals Industry, 2023, 55(6): 50-56. |
| [5] | TENG Jiayang, FENG Qingge, ZHANG Xuan, QIN Fanghong, FENG Jinghang, HU Jiawen, CHEN Chaohong. Study on preparation of pseudo-boehmite from aluminum dross resource treatment [J]. Inorganic Chemicals Industry, 2023, 55(11): 130-138. |
| [6] | ZHANG Zhiqiang, CUI Kangping, CHEN Xing, LI Haiyang. Optimization of preparation of ultrafine chromium oxide by carbothermal reduction of sodium dichromate by response surface methodology [J]. Inorganic Chemicals Industry, 2023, 55(10): 136-144. |
| [7] | FANG Weicheng,CHENG Xingxing,SUN Changrong. Optimization of preparation of sludge/fly ash composite ceramsite filler materials by response surface methodology [J]. Inorganic Chemicals Industry, 2022, 54(9): 119-125. |
| [8] | FU Ziqi, ZHANG Cheng, SHENG Yong, JI Lijun. Study on preparation of phosphoric acid by leaching fluoride residue from wet-process phosphoric acid with organic solvents [J]. Inorganic Chemicals Industry, 2022, 54(7): 129-134. |
| [9] | LI Yajiao,ZHAO Yiwei,JU Kai,TANG Renlong,LI Longqing,SHAO Xiaoping,ZHANG Gaofeng,REN Wuang. Study on optimization of extraction conditions in process of determination of ammonia content in fly ash based on response surface method [J]. Inorganic Chemicals Industry, 2022, 54(4): 145-151. |
| [10] | LI Yalin,HUANG Yu,Li Dongao,SUI Zhaoyi,HE Haiyang,LIU Haozhao. Preparation of Fe-impregnated biochar from food waste by solvothermal method [J]. Inorganic Chemicals Industry, 2022, 54(11): 96-103. |
| [11] | SHI Chenglong,MA Shuqing,QIN Yaru,LIU Bing,LI Haichao. Study on optimization of synergistic lithium extraction process of tributyl phosphate-ethyl butyrate system by response surface method [J]. Inorganic Chemicals Industry, 2022, 54(10): 37-41. |
| [12] | Wang Kuangbin,Xu Shengxia,Wang Yongqin. Research on purification process of lithium difluorosulfimide optimized by response surface method [J]. Inorganic Chemicals Industry, 2020, 52(9): 62-65. |
| [13] | Huang Jiming,Liu Runqing,Wu Sizhan,Song Juan. Optimization of extraction process of chlorination roasting-water leaching process for low-grade rhodochrosite by response surface methodology [J]. Inorganic Chemicals Industry, 2019, 51(3): 34-37. |
| [14] | CHEN Bo, SONG Xing-Fu, XU Yan-Xia, SUN Yu-Zhu, YU Jian-Guo. Optimization of preparation of calcium carbonate from continuous crystallization process of ammonium carbonate and conversion of calcium sulfate by response surface methodology [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(9): 18-. |
| [15] | LONG Yun-Fei, ZHANG Yu, SU Jing, 吕Xiao-Yan , WEN Yan-Xuan. Response surface optimization of process parameters of silver leached from zinc-smelting slag by modified thiourea method [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(1): 13-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||