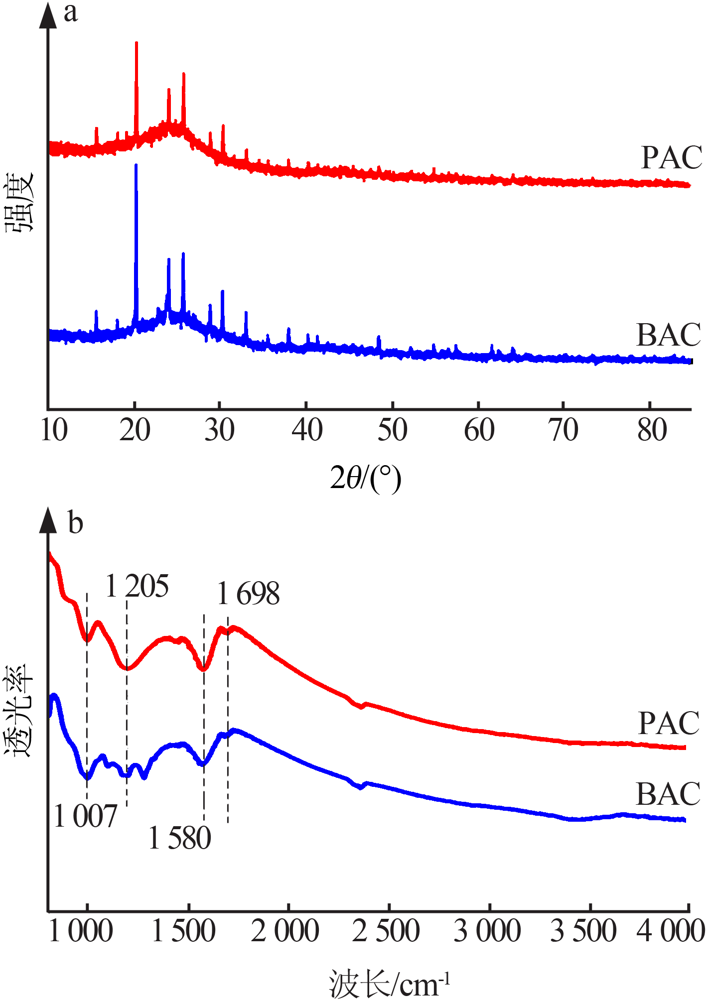
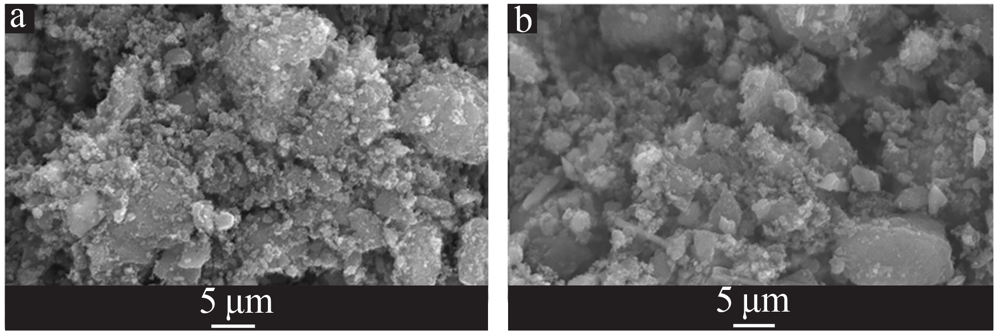
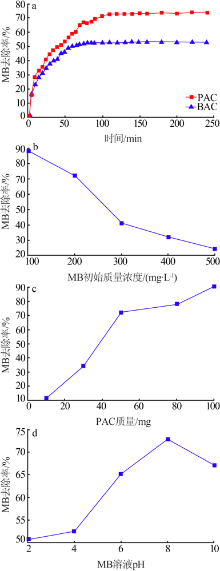
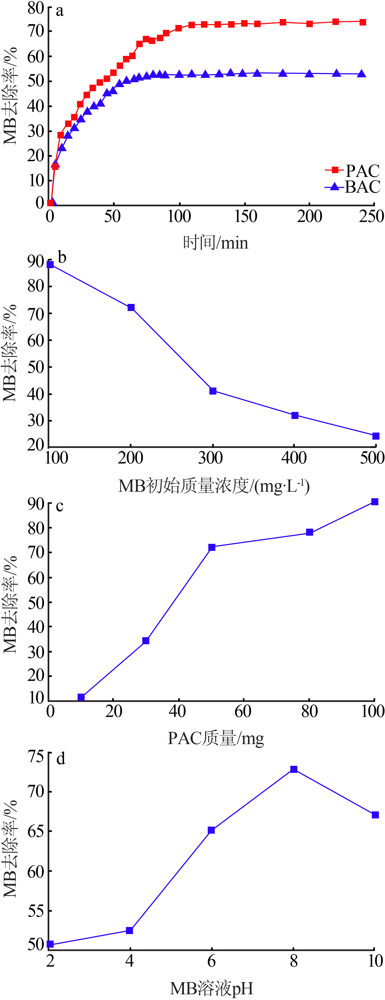
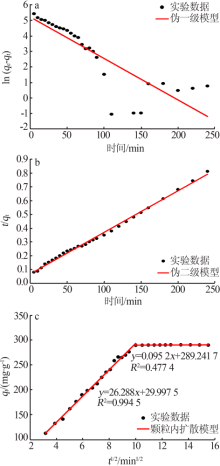
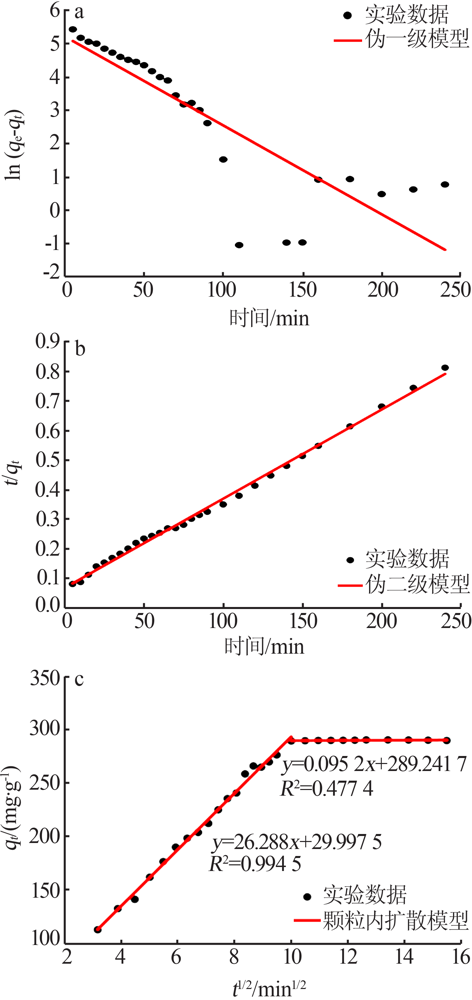
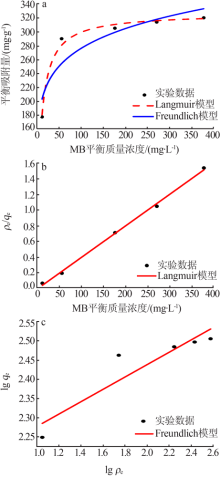
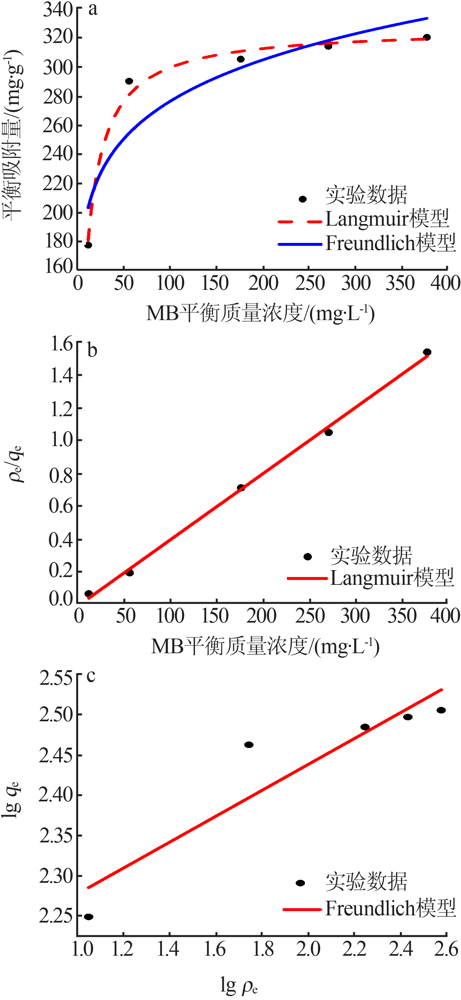
| [1] |
DAI Yingjie, SUN Qiya, WANG Wensi, et al. Utilizations of agricul- tural waste as adsorbent for the removal of contaminants:A revi- ew[J]. Chemosphere, 2018, 211:235-253.
doi: S0045-6535(18)31254-2
pmid: 30077103
|
| [2] |
AGUAYO-VILLARREAL I A, BONILLA-PETRICIOLET A, MUIZ- VALENCIA R. Preparation of activated carbons from pecan nutshell and their application in the antagonistic adsorption of heavy metal ions[J]. Journal of Molecular Liquids, 2017, 230:686-695.
doi: 10.1016/j.molliq.2017.01.039
|
| [3] |
YUNUS Z M, OTHMAN N, AL-GHEETHI A, et al. Adsorption of heavy metals from mining effluents using honeydew peels activated carbon;isotherm,kinetic and column studies[J]. Journal of Disper- sion Science and Technology, 2020, 42(5):715-729.
|
| [4] |
DEMIRAL H, GÜNG C R. Adsorption of copper(Ⅱ) from aqueous solutions on activated carbon prepared from grape bagasse[J]. Journal of Cleaner Production, 2016, 124:103-113.
doi: 10.1016/j.jclepro.2016.02.084
|
| [5] |
SUN Yuanyuan, LI Hong, LI Guangci, et al. Characterization and ciprofloxacin adsorption properties of activated carbons prepared from biomass wastes by H3PO4 activation[J]. Bioresource Technolo- gy, 2016, 217:239-244.
|
| [6] |
SHANG Jingge, HE Wei, FAN Chengxin. Adsorption of dimethyl tri- sulfide from aqueous solution on a low-cost adsorbent:Thermally ac- tivated pinecone[J]. Chinese Journal of Oceanology and Limnology, 2015, 33(1):169-175.
doi: 10.1007/s00343-015-4085-y
|
| [7] |
DJELLOUL C, HAMDAOUI O. Dynamic adsorption of methylene blue by melon peel in fixed-bed columns[J]. Desalination & Water Treatment, 2015, 56(11):2966-2975.
|
| [8] |
ZHOU Yong, ZHANG Lei, CHENG Zhengjun. Removal of organic pollutants from aqueous solution using agricultural wastes:A review[J]. Journal of Molecular Liquids, 2015, 212:739-762.
doi: 10.1016/j.molliq.2015.10.023
|
| [9] |
王志康, 黄路婷, 范娟娟, 等. α-Fe2O3活性炭的制备以及对亚甲基蓝去除影响[J]. 无机盐工业, 2019, 51(2):75-78,83.
|
| [10] |
SHUANG Jiao, ZHAO Yiming, BI Meng, et al. Removal of methy- lene blue from water by BiFeO3/carbon fibre nanocomposite and its photocatalytic regeneration[J]. Catalysts, 2018, 8(7). Doi: 10.3390/catal8070267.
doi: 10.3390/catal8070267
|
| [11] |
HUANG Yang, LI Shunxing, CHEN Jianhua, et al. Adsorption of Pb(Ⅱ) on mesoporous activated carbons fabricated from water hyacinth using H3PO4 activation:Adsorption capacity,kinetic and isotherm studies[J]. Applied Surface Science, 2014, 293:160-168.
doi: 10.1016/j.apsusc.2013.12.123
|
| [12] |
ZUBRIK A, MATIK M, HREDZAK S, et al. Preparation of chemi- cally activated carbon from waste biomass by single-stage and two-stage pyrolysis[J]. Journal of Cleaner Production, 2017, 143:643-653.
doi: 10.1016/j.jclepro.2016.12.061
|
| [13] |
ZHANG Ge, SHI Li, ZHANG Yongfang, et al. Aerobic granular slu- dge-derived activated carbon:Mineral acid modification and su- perior dye adsorption capacity[J]. RSC Advances, 2015, 5(32):25279-25286.
doi: 10.1039/C4RA15216F
|
| [14] |
TAN I A, AHMAD A L, HAMEED B H. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium,kinetic and thermodynamic studies[J]. Journal of Hazardous Materials, 2008, 154(1):337-346.
doi: 10.1016/j.jhazmat.2007.10.031
|
| [15] |
OZER C, IMAMOGLU M, TURHAN Y, et al. Removal of methy lene blue from aqueous solutions using phosphoric acid activated carbon produced from hazelnut husks[J]. Toxicological & Environmental Chemistry Reviews, 2012, 94(7/8):1283-1293.
|
| [16] |
MAIA L S, SILVA A, CARNEIRO E S, et al. Activated carbon from palm fibres used as an adsorbent for methylene blue removal[J]. Journal of Polymers and the Environment, 2021, 29:1162-1175.
doi: 10.1007/s10924-020-01951-0
|
| [17] |
CHEN Congjin, MI Shuai, LAO Dongmei, et al. Single-step synthe- sis of eucalyptus sawdust magnetic activated carbon and its adso- rption behavior for methylene blue[J]. RSC Advances, 2019, 9(39):22248-22262.
doi: 10.1039/c9ra03490k
|
| [18] |
BOUNAAS M, BOUGUETTOUCHA A, CHEBLI D, et al. Role of the wild carob as biosorbent and as precursor of a new high-sur- face-area activated carbon for the adsorption of methylene blue[J]. Arabian Journal for Science and Engineering, 2021, 46:325-341.
doi: 10.1007/s13369-020-04739-5
|
 ),XU Rongsheng1,2(
),XU Rongsheng1,2( ),LI Mei1,2,ZHANG Haiyong3
),LI Mei1,2,ZHANG Haiyong3