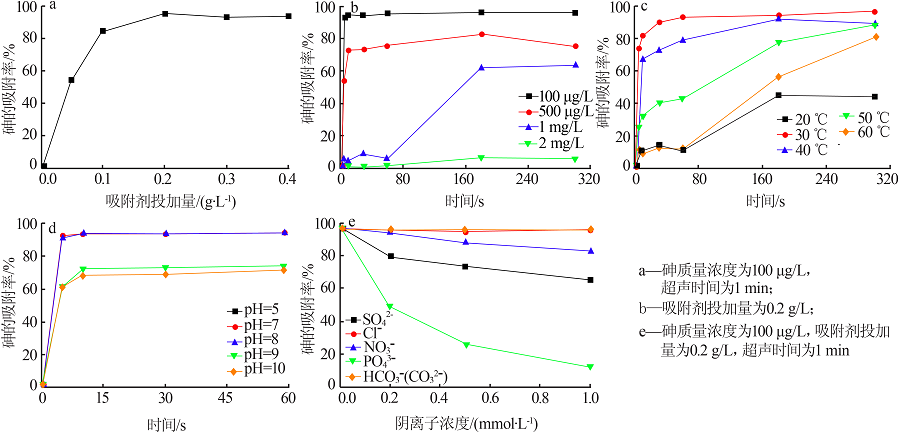
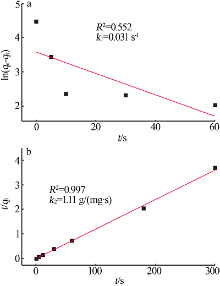
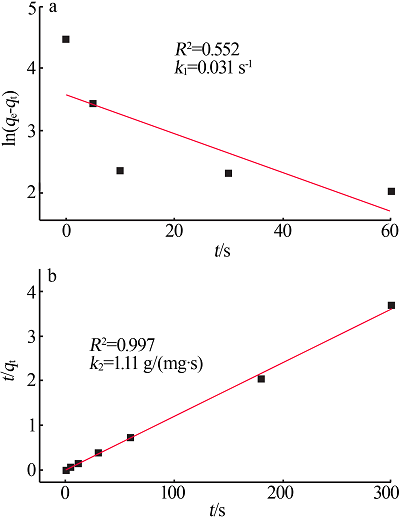
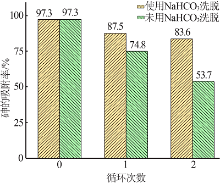
Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (3): 102-108.doi: 10.19964/j.issn.1006-4990.2021-0267
• Environment·Health·Safety • Previous Articles Next Articles
Enhanced effect of ultrasound on adsorption of inorganic arsenic species from wastewater by FeMn oxides
- School of Chemistry and Chemical Engineering,Inner Mongolia University of Science & Technology,Baotou 014010,China
-
Received:2021-05-26Online:2022-03-10Published:2022-03-18 -
Contact:GUO Xiaohui E-mail:857974721@qq.com;gxhsxicc@163.com
CLC Number:
Cite this article
TAN Wenxi,GUO Xiaohui. Enhanced effect of ultrasound on adsorption of inorganic arsenic species from wastewater by FeMn oxides[J]. Inorganic Chemicals Industry, 2022, 54(3): 102-108.
share this article
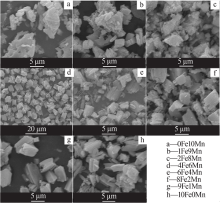
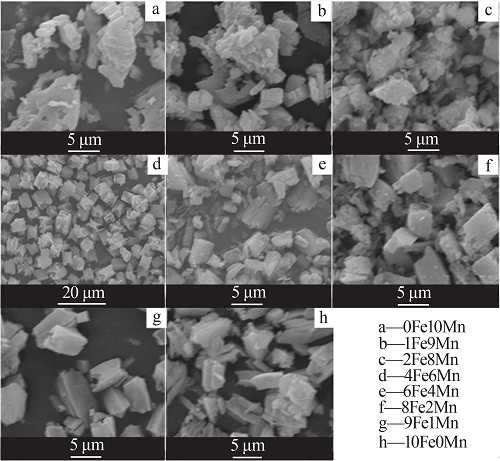
Table 1
Name of materials with different Fe/Mn molar ratios"
| 样品名称 | m(FeCl2· 4H2O)/g | m(MnCl2· 4H2O)/g | 样品名称 | m(FeCl2· 4H2O)/g | m(MnCl2· 4H2O)/g |
|---|---|---|---|---|---|
| 0Fe10Mn | 0.000 | 15.833 | 6Fe4Mn | 9.940 | 5.937 |
| 1Fe9Mn | 1.390 | 12.468 | 8Fe2Mn | 11.929 | 2.968 |
| 2Fe8Mn | 2.982 | 11.875 | 9Fe1Mn | 12.525 | 1.385 |
| 4Fe6Mn | 6.958 | 9.896 | 10Fe0Mn | 15.905 | 0.000 |
Table 1
Table 2
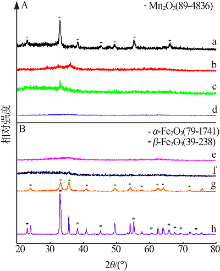
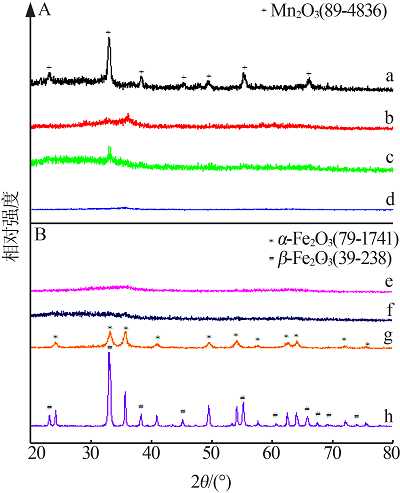
Secific surface area,mean pore size and pore volume of FeMn composite oxides"
| 样品 | 比表面积/ (m2·g-1) | 平均孔径/ nm | 孔体积/ (cm3·g-1) |
|---|---|---|---|
| 0Fe10Mn | 53.8 | 18.7 | 0.24 |
| 1Fe9Mn | 226.8 | 6.2 | 0.34 |
| 2Fe8Mn | 276.4 | 5.2 | 0.34 |
| 4Fe6Mn | 257.7 | 4.1 | 0.29 |
| 6Fe4Mn | 396.6 | 3.7 | 0.34 |
| 8Fe2Mn | 324.0 | 4.3 | 0.34 |
| 9Fe1Mn | 184.7 | 6.6 | 0.32 |
| 10Fe0Mn | 147.6 | 7.1 | 0.27 |
Table 2
| [1] |
HUO J, XU L, CHEN X, et al. Direct epitaxial synjournal of magnetic Fe3O4@UiO-66 composite for efficient removal of arsenate from wa-ter[J]. Microporous and Mesoporous Materials, 2019, 276:68-75.
doi: 10.1016/j.micromeso.2018.09.017 |
| [2] | 童宏祥. 高浓度含砷污泥氧化稳定化处理与中试研究[D]. 武汉:华中科技大学, 2016. |
| [3] | 高艳芳, 何云. 砷暴露的心血管毒性效应及其机制研究进展[J]. 毒理学杂质, 2016, 30(6):469-471,474. |
| [4] | 金银龙, 梁超轲, 何公理, 等. 中国地方性砷中毒分布调查(总报告)[J]. 卫生研究, 2003, 32(6):519-540. |
| [5] |
ALKURDI S S A, HERATH I, BUNDSCHUH J, et al. Biochar versus bone char for a sustainable inorganic arsenic mitigation in water:What needs to be done in future research[J]. Environment International, 2019, 127:52-69.
doi: 10.1016/j.envint.2019.03.012 |
| [6] | 张伟. 新型复合铁钛锰吸附剂的研制及其除砷效能与机制研究[D]. 哈尔滨:哈尔滨工业大学, 2019. |
| [7] | XU F, CHEN H, DAI Y, et al. Arsenic adsorption and removal by a new starch stabilized ferromanganese binary oxide in water[J]. Jo-urnal of Environmental Management, 2019, 245:160-167. |
| [8] |
CHENG Y, ZHANG S, HUANG T, et al. Arsenite removal from groun-undwater by iron-manganese oxides filter media:Behavior and me-chanism[J]. Water Environment Research, 2019, 91(6):536-545.
doi: 10.1002/wer.2019.91.issue-6 |
| [9] |
FOROUTAN R, MOHAMMADI R, ADELEYE A S, et al. Efficient arsenic(V) removal from contaminated water using natural clay and clay composite adsorbents[J]. Environmental Science and Pollution Research, 2019, 26(4):29748-29762.
doi: 10.1007/s11356-019-06070-5 |
| [10] | SEID-MOHAMMADI A, ASGARI G, RAHMANI A, et al. Synjournal and application of iron/copper bimetallic nanoparticles doped nat-ural zeolite composite coupled with ultrasound for removal of arse-nic(Ⅲ) from aqueous solutions[J]. Desalination and Water Treat-ment, 2019, 161:343-353. |
| [11] |
TANAKA Y, OKAWA H, TAKAHASHI Y Y, et al. Utilization of la-yered double hydroxide to remove arsenic and suppress pH decre-ment during ultrasound oxidation of arsenious acid[J]. Japanese Jo-urnal of Applied Physics, 2018, 57(7S1).Doi: 10.7567/JJAP.57.07LE02.
doi: 10.7567/JJAP.57.07LE02 |
| [12] |
PAN S Z, JIN C Z, YANG X A, et al. Ultrasound enhanced solid-phase extraction of ultra-trace arsenic on Fe3O4@AuNPs magnetic particles[J]. Talanta, 2020, 209.Doi: 10.1016/j.talanta.2019.120553.
doi: 10.1016/j.talanta.2019.120553 |
| [13] |
GUO X, LIANG X, ZHOU X, et al. Structural,magnetic and electro-chemical properties of hierarchical microspindle-like Fe2-xMnxO3 composites from thermal decomposition of oxalates[J]. Journal of Materials Science:Materials in Electronics, 2020, 31(5):3986-3995.
doi: 10.1007/s10854-020-02946-2 |
| [14] |
HOU L, HUA H, LIAN L, et al. Green template-free synjournal of hi-erarchical shuttle-shaped mesoporous ZnFe2O4 microrods with en-hanced lithium storage for advanced Li-ion batteries[J]. Chemistry:A European Journal, 2015, 21(37):13012-13019.
doi: 10.1002/chem.201501876 |
| [15] | 张杰, 白永波, 郭小惠. 铁锰复合氧化物微米棒:合成中的溶剂效应及其砷吸附性能[J]. 人工晶体学报, 2019, 48(12):2338-2343. |
| [16] |
CHEN S, KIMATU B M, FANG D, et al. Effect of ultrasonic treat-ment on transformations of arsenic species in edible mushrooms[J]. Analytical Letters, 2020, 53(1):102-121.
doi: 10.1080/00032719.2019.1639056 |
| [17] | SMEDLEY P L, KINNIBURGH D G. A review of the source,beha-viour and distribution of arsenic in natural waters[J]. Applied Geo-chemistry, 2002, 17(5):517-568. |
| [18] |
DOJCINOVIC B P, JANCAR B, BESSAIS L, et al. Differently shaped nanocrystalline(Fe,Y)3O4 and its adsorption efficiency toward in-organic arsenic species[J]. Nanotechnology, 2019, 30(47).Doi: 10.1088/1361-6528/ab3ca2.
doi: 10.1088/1361-6528/ab3ca2 |
| [19] |
WEN Z, LU J, ZHANG Y, et al. Facile inverse micelle fabrication of magnetic ordered mesoporous iron cerium bimetal oxides with exce-llent performance for arsenic removal from water[J]. Journal of Hazardous Materials, 2020, 383.Doi: 10.1016/j.jhazmat.2019.121172.
doi: 10.1016/j.jhazmat.2019.121172 |
| [20] |
REN Z, ZHANG G, CHEN J P. Adsorptive removal of arsenic from water by an iron-zirconium binary oxide adsorbent[J]. Journal of Colloid and Interface Science, 2011, 358(1):230-237.
doi: 10.1016/j.jcis.2011.01.013 |
| [21] | LI Z, LIU X, JIN W, et al. Adsorption behavior of arsenicals on MIL-101(Fe):The role of arsenic chemical structures[J]. Journal of Co-lloid Interface Science, 2019, 554:692-704. |
| [22] |
LEE S Y, JUNG K W, CHOI J W, et al. In situ synjournal of hierarchi-cal cobalt-aluminum layered double hydroxide on boehmite surface for efficient removal of arsenate from aqueous solutions:Effects of solution chemistry factors and sorption mechanism[J]. Chemical Engineering Journal, 2019, 368:914-923.
doi: 10.1016/j.cej.2019.03.043 |
| [1] | XU Mengyao, ZHANG Xin, HE Kunpeng, HE Jian, JIANG Wei. Preparation of yttrium oxide and zirconium phosphate adsorbents from zirconium-yttrium waste and evaluation of their performance [J]. Inorganic Chemicals Industry, 2024, 56(3): 116-124. |
| [2] | LI Qiaoyun, HUANG Xiuxing, WEI Wenye, CHEN Zhen. Study on adsorption of methylene blue by activated carbon with acid/alkali synergistically modified fly ash [J]. Inorganic Chemicals Industry, 2024, 56(3): 131-136. |
| [3] | LI Yang, LOU Feijian, SUI Xin, LI Keyan, LIU Fei, GUO Xinwen. Preparation of amine-functionalized fumed SiO2 materials and their performance for CO2 adsorption [J]. Inorganic Chemicals Industry, 2024, 56(2): 38-43. |
| [4] | ZHANG Li, ZHANG Dan, PAN Hongyan, DONG Yonggang, LI Wenfei, QIN Hong. Study on preparation of low ash activated carbon by phosphoric acid method [J]. Inorganic Chemicals Industry, 2024, 56(2): 95-103. |
| [5] | YAN Zhen, QIU Zhaofu, JIN Xibiao, WANG Yuan, LIU Chang, YANG Ji. Study on 4A molecular sieve loaded with Ce and γ-Fe2O3 for removal of Sb(Ⅲ) and Sb(Ⅴ) in water [J]. Inorganic Chemicals Industry, 2024, 56(1): 81-89. |
| [6] | ZHOU Shiqi, WANG Tao, JING Fangli, LUO Shizhong. Study on performance of magnesium nitrate-modified carbon molecular sieve for separation of nitrogen/methane [J]. Inorganic Chemicals Industry, 2023, 55(9): 75-80. |
| [7] | LI Chao, WANG Liping, DAI Yin, GAO Guimei, ZHANG Yunfeng, HONG Yu, XU Lijun, CUI Yongjie. Study on alkali fusion hydrothermal synthesis of 13X zeolite from high silicon tailings and its adsorption on lead,copper and zinc ions [J]. Inorganic Chemicals Industry, 2023, 55(9): 88-93. |
| [8] | WANG Yingnan, SHENG Linlin, HUANG Juan, HUANG Zhanbin. Study on adsorption performance of lead from water by coal-fired slag [J]. Inorganic Chemicals Industry, 2023, 55(8): 109-115. |
| [9] | TANG Yifu, CAO Changchun, LÜ Peng. Study on adsorption of cadmium-humic acid by hydroxyiron oxide [J]. Inorganic Chemicals Industry, 2023, 55(8): 124-131. |
| [10] | HAN Hongjing, ZHANG Jingze, LAMAO Zhuoma, HAN Jizhe, WU Yongmin, TANG Weiping. Preparation of Al-Co co-doped lithium manganese oxide and its adsorption performance of lithium [J]. Inorganic Chemicals Industry, 2023, 55(7): 38-44. |
| [11] | FAN Fangfang, TONG Zhongkai, ZUO Weiyuan. Study on adsorption of tetracycline from wastewater by calcium modified peanut shell biochar [J]. Inorganic Chemicals Industry, 2023, 55(6): 109-115. |
| [12] | TIAN Yuling, CHENG Yang, HAN Rong, ZHOU Mei, WANG Chengjie, GE Qiangru. Effect of CaO on heavy metals stability and adsorption properties of sludge-derived biochar [J]. Inorganic Chemicals Industry, 2023, 55(6): 124-129. |
| [13] | FU Minglian, CEN Jianmei, CHEN Zhangxu. Study on preparation of magnetic SiO2/chitosan composite aerogel and its adsorption for Cu2+ [J]. Inorganic Chemicals Industry, 2023, 55(6): 70-77. |
| [14] | XU Chunhui, WANG Feng, LING Changjian, WANG Zirui, TANG Zhongfeng. Research progress of CO2 capture by metal oxides modified by molten salts [J]. Inorganic Chemicals Industry, 2023, 55(5): 1-7. |
| [15] | LIU Zihan, XI Guojun, LEI Guangping. Application of MOFs in adsorption refrigeration/heat pump [J]. Inorganic Chemicals Industry, 2023, 55(4): 20-26. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||