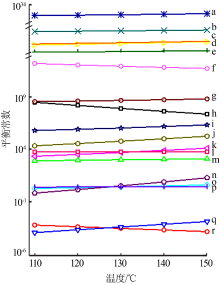
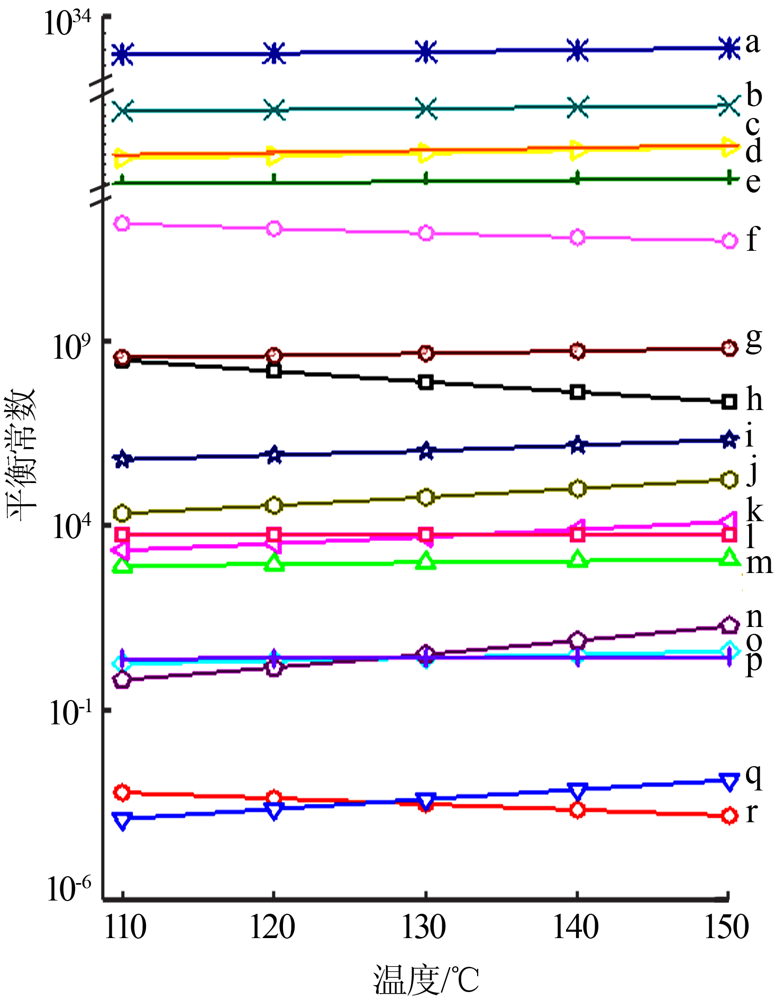
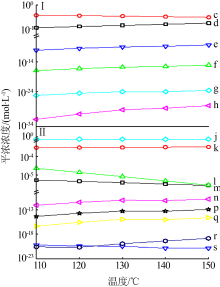
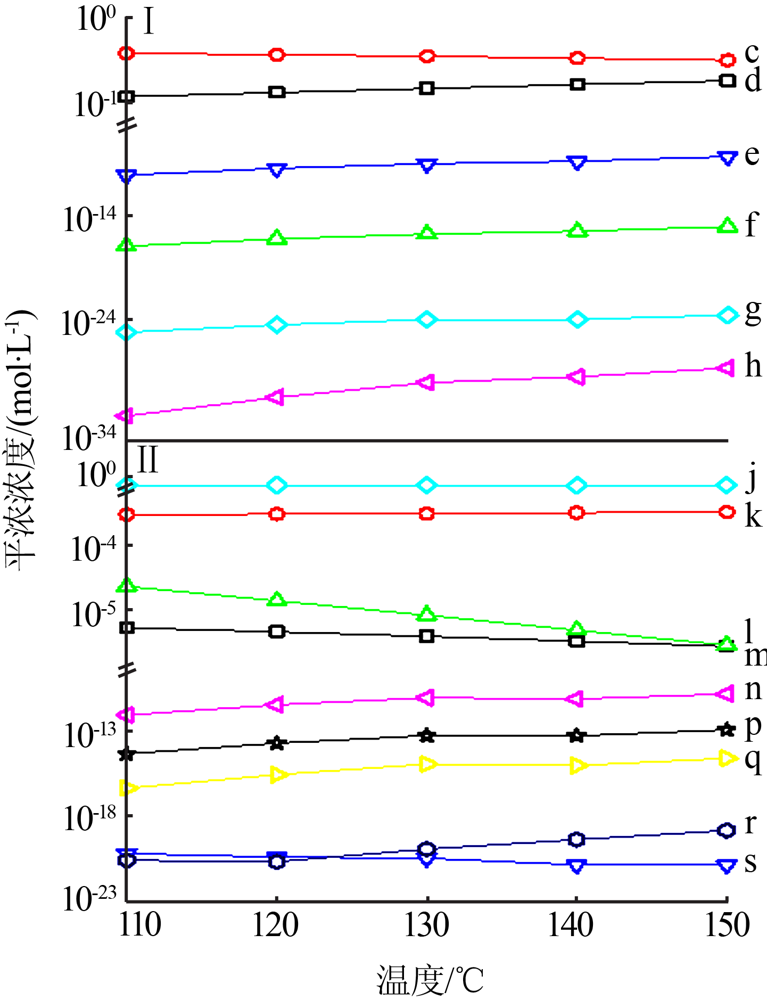
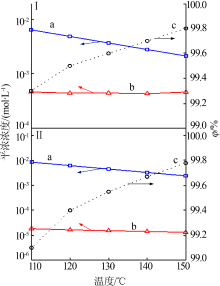
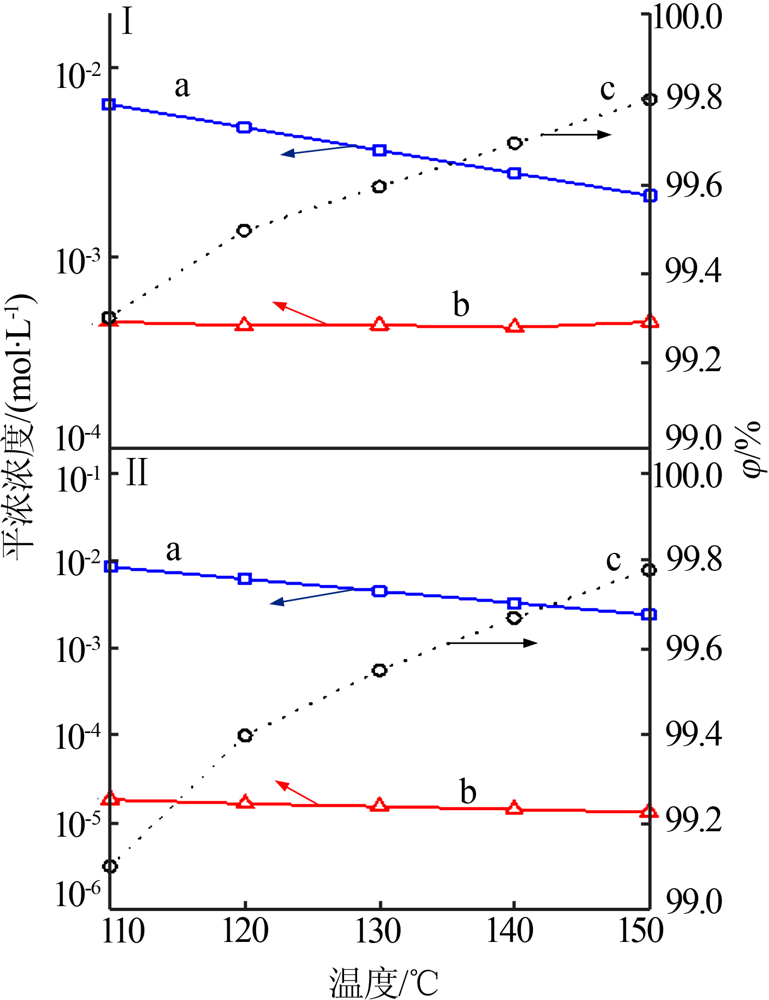
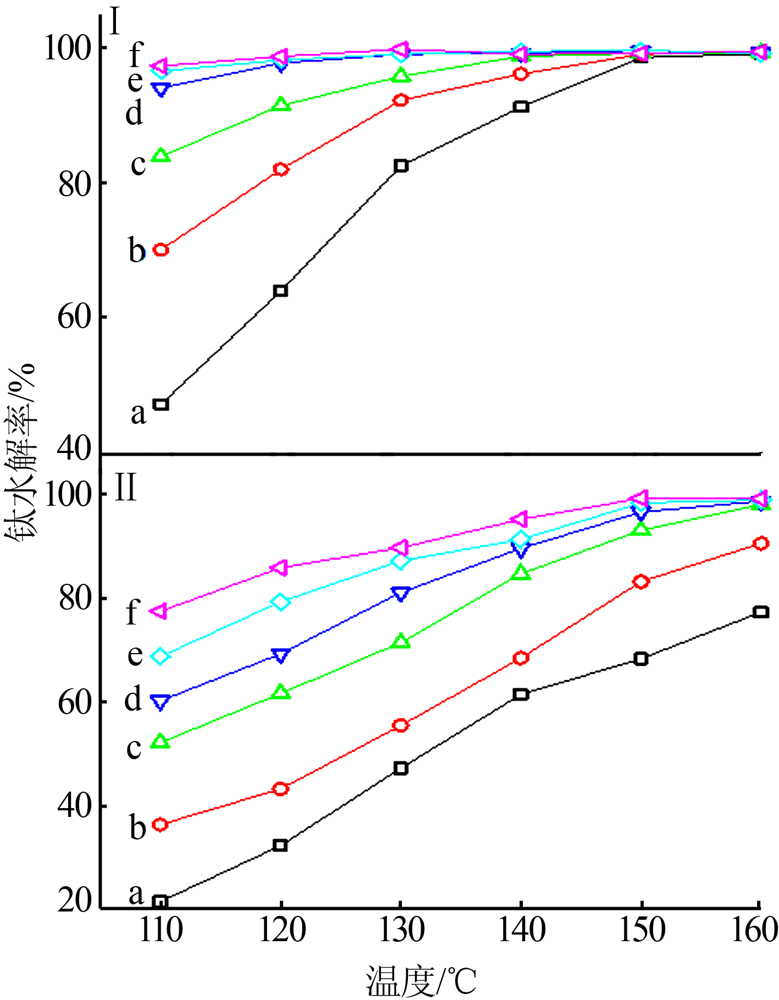
| [1] |
刘帅, 张宗旺, 张建良, 等. 高钛型高炉渣钛提取工艺研究现状及发展展望[J]. 中国冶金, 2020, 30(3):1-7.
|
| [2] |
吴胜利. 高钛高炉渣综合利用的研究进展[J]. 中国资源综合利用, 2013, 31(2):39-43.
|
| [3] |
郝百川, 李子越, 贾东方, 等. 含钛高炉渣的综合利用[J]. 矿产综合利用, 2020(6):1-6.
|
| [4] |
黄家旭, 杨仰军, 陆平, 等. 攀钢碳化高炉渣低温氯化试验研究[J]. 钢铁钒钛, 2011, 32(4):12-15,50.
|
| [5] |
张利凡, 丁满堂, 何翠萍, 等. 含钛高炉渣火法提钛[J]. 中国资源综合利用, 2020, 38(10):94-96.
|
| [6] |
何思祺, 孙红娟, 彭同江, 等. 碱法处理含钛高炉渣的矿相变化及工艺条件探索[J]. 钢铁钒钛, 2015, 36(6):44-50,56.
|
| [7] |
李鑫, 于洪浩, 张侯芳, 等. 熔盐高效分解含钛高炉渣制备纳米二氧化钛[J]. 化工学报, 2015, 66(2):827-833.
|
| [8] |
BIAN Z Z, FENG Y L, HAO-RAN L I. Extraction of valuable metals from Ti-bearing blast furnace slag using ammonium sulfate pressu- rized pyrolysis-acid leaching processes[J]. Transactions of Nonfer- rous Metals Society of China, 2020, 30(10):2836-2847.
|
| [9] |
刘维燥, 胡金鹏, 刘清才, 等. 硫酸铵与钛酸钙焙烧动力学[J]. 化工进展, 2021, 40(8):4624-4630.
|
| [10] |
HE S, PENG T, SUN H. Titanium recovery from Ti-bearing blast furnace slag by alkali calcination and acidolysis[J]. JOM, 2019, 71(9):3196-3201.
doi: 10.1007/s11837-019-03575-9
|
| [11] |
曹洪杨, 付念新, 康常波, 等. 改性含钛高炉渣的盐酸加压浸出[J]. 矿产综合利用, 2008(4):11-14.
|
| [12] |
熊瑶, 李春, 梁斌, 等. 盐酸浸出自然冷却含钛高炉渣[J]. 中国有色金属学报, 2008, 18(3):557-563.
|
| [13] |
熊瑶, 梁斌, 李春. 自然冷却含钛高炉渣中钛的提取与分离[J]. 过程工程学报, 2008, 8(6):1092-1097.
|
| [14] |
薛鑫, 李万博, 王建伟, 等. 含钛高炉渣钛提取中酸解率影响因素的研究[J]. 金属矿山, 2009(3):178-181.
|
| [15] |
陈启福, 张燕秋, 方民宪, 等. 攀钢高炉渣提取二氧化钛及三氧化二钪的研究[J]. 钢铁钒钛, 1991, 12(3):30-35.
|
| [16] |
YANG Zhuoying, YANG Fan, YI Meigui, et al. Estimation of reac- tion heat in Ti-bearing blast furnace slag-sulfuric acid system based on mechanical mixture model[J]. Mining Metallurgy & Ex- ploration, 2021, 38(3):1-6.
|
| [17] |
刘晓华, 隋智通. 钛渣酸解液制取水合TiO2及杂质行为研究[J]. 矿产综合利用, 2005(6):12-16.
|
| [18] |
彭兵, 易文质, 彭及, 等. 复杂硫酸盐溶液体系水解制取钛白的热力学研究[J]. 湖南有色金属, 1997(2):47-48.
|
| [19] |
陈朝华, 刘长河. 钛白粉生产及应用技术[M]. 北京: 化学工业出版社, 2006.
|
| [20] |
裴润. 硫酸法钛白生产[M]. 北京: 化学工业出版社, 1982.
|
| [21] |
张益都. 硫酸法钛白粉生产技术创新[M]. 北京: 化学工业出版社, 2010.
|
| [22] |
叶大伦. 实用无机物热力学数据手册[J]. 北京: 冶金工业出版社, 1981.
|
| [23] |
J.A.迪安. 兰氏化学手册[M]. 魏俊发译.2版.北京: 科学出版社, 2003.
|
 ),YANG Fan2,YI Meigui1(
),YANG Fan2,YI Meigui1( ),XIANG Lan2(
),XIANG Lan2( )
)