| [1] |
MUHAMMAD Saeed Akram, MUHAMMAD Ashraf. Exogenous app-lication of potassium dihydrogen phosphate can alleviate the adverse effects of salts stress on sunflower[J]. Journal of Plant Nutrition, 2011, 34:1041-1057.
doi: 10.1080/01904167.2011.555585
|
| [2] |
王东头, 刘长青, 王拥军. 磷酸二氢钾在农业上的超常量施用[J]. 磷肥与复肥, 2017, 32(10):34-36.
|
| [3] |
吴宇川, 何兵兵, 薛绍秀, 等. 磷酸二氢钾的制备与应用研究进展[J]. 磷肥与复肥, 2017, 32(2):30-34.
|
| [4] |
郑雯佳, 王辛龙, 张志业, 等. 溶析结晶法制备磷酸二氢钾[J]. 磷肥与复肥, 2018, 33(5):16-18,39.
|
| [5] |
BARATA P A, SERRANO M L. Salting-out precipitation of potassi-um dihydrogen phosphate(KDP) Ⅲ.Growth process[J]. Journal of Crystal Growth, 1998, 194:101-108.
doi: 10.1016/S0022-0248(98)00655-1
|
| [6] |
HAN Bing, SHA Zuoliang, PARTANENA J I, et al. Solubility of po-tassium dihydrogen phosphate in aqueous solutions of acetone,ethyl acetate,and thiourea from T=(298.15~313.15) K[J]. Fluid Phase Equilibria, 2012, 336:16-21.
doi: 10.1016/j.fluid.2012.08.031
|
| [7] |
BARATA P A, SERRANO M L. Thermodynamic representation of the solubility for potassium dihydrogen phosphate(KDP)+water+alcohols systems[J]. Fluid Phase Equilibria, 1997, 141:247-263.
doi: 10.1016/S0378-3812(97)00188-X
|
| [8] |
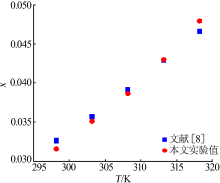
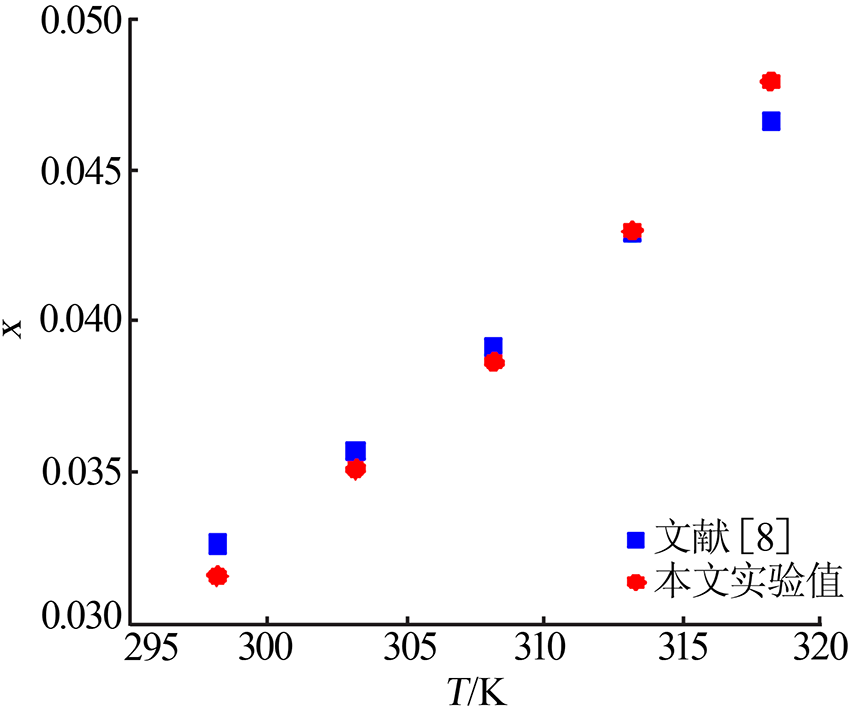
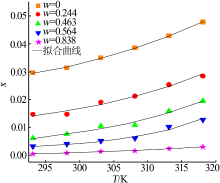
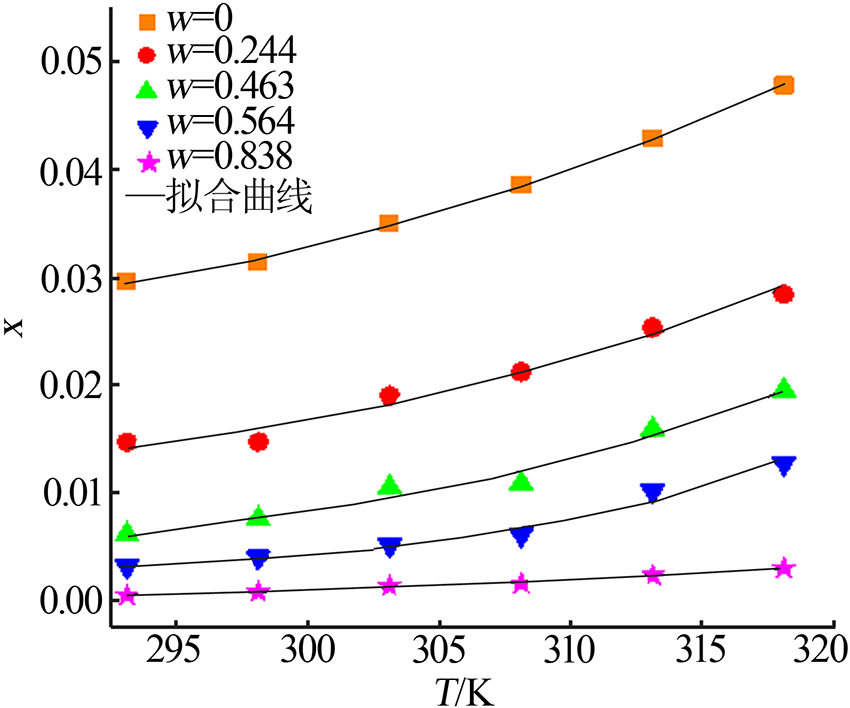
ZHOU Xiaohou, ZHENG Wenjia, XU Dehua, et al. Solubility meas-urement and thermodynamics modelling for potassium dihydrogen phosphate in a water-ethanol system from 293.2 to 323.2 K[J]. Fluid Phase Equilibria, 2020, 512.Doi: 10.1016/j.fluid.2020.112533.
doi: 10.1016/j.fluid.2020.112533
|
| [9] |
雷杰. 溶析结晶法处理煤化工含Na+高浓盐水的研究[D]. 北京:北京化工大学, 2017.
|
| [10] |
XU Dejun, XIONG Xing, YANG Lin, et al. Determination of the so-lubility of ammonium dihydrogen phosphate in water-ethanol sy-stem at different temperatures from 283.2 to 343.2 K[J]. Journal of Chemical & Engineering Date, 2015, 61(1):78-82.
|
| [11] |
陈丽芳, 张勤勤, 林田, 等. 硼酸在氯化钙溶液中溶解度的测定与关联[J]. 无机盐工业, 2019, 51(5):70-73.
|
| [12] |
XU Renjie, ZHENG Min, ALI Farajtabar, et al. Solubility modeling and preferential solvation of adenine in solvent mixtures of (N,N-dimethylformamide,N-methyl pyrrolidone,propylene glycol and dimethyl sulfoxide) plus water[J]. The Journal of Chemical Ther-modynamics, 2018, 215:225-234.
|
 ),CHENG Zhuo,TANG Xiuhua,ZHANG Fengzhen(
),CHENG Zhuo,TANG Xiuhua,ZHANG Fengzhen( )
)