Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (12): 85-90.doi: 10.19964/j.issn.1006-4990.2021-0097
• Research & Development • Previous Articles Next Articles
Measurement and correlation of solubility of magnesium chloride hexahydrate in six polyols
CHEN Yunfei1( ),MAO Lili1,LIU Dan1,WANG Haizeng1,2(
),MAO Lili1,LIU Dan1,WANG Haizeng1,2( )
)
- 1. College of Chemistry and Chemical Engineering,Ocean University of China,Qingdao 266100,China
2. Shandong Ocean Chemical Industry Scientific Research Institute
-
Received:2021-02-18Online:2021-12-10Published:2021-12-16 -
Contact:WANG Haizeng E-mail:15194290058@163.com;haizwang@ouc.edu.cn
CLC Number:
Cite this article
CHEN Yunfei,MAO Lili,LIU Dan,WANG Haizeng. Measurement and correlation of solubility of magnesium chloride hexahydrate in six polyols[J]. Inorganic Chemicals Industry, 2021, 53(12): 85-90.
share this article
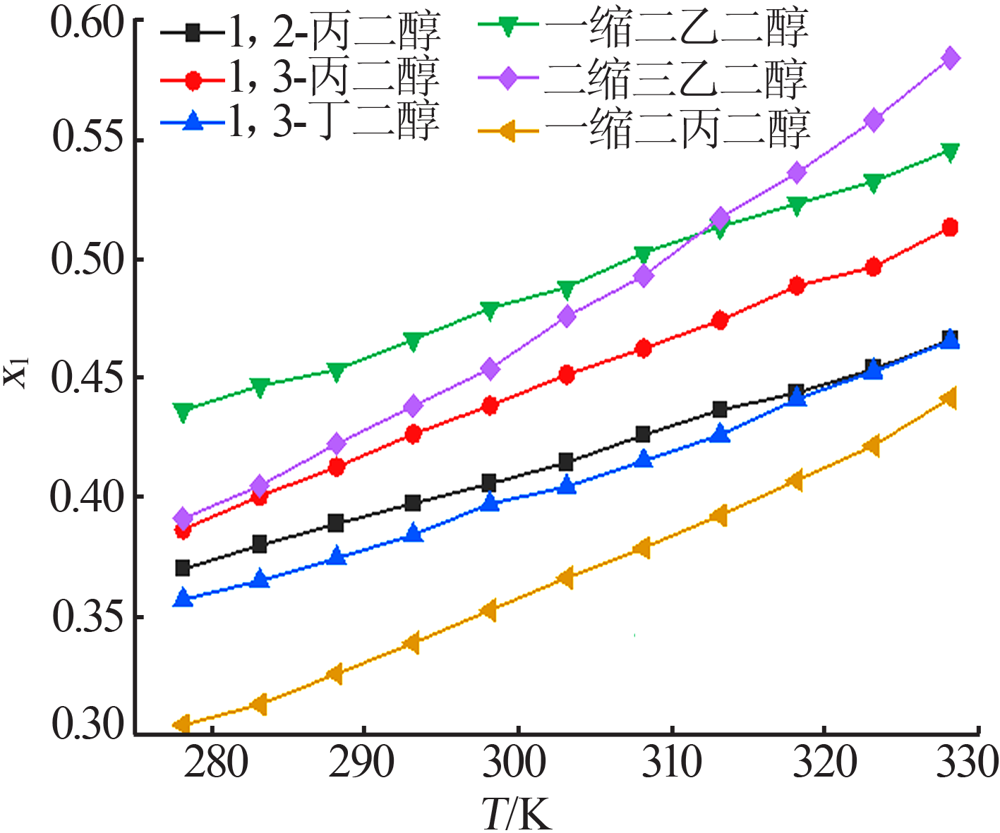
Table 1
The molar solubility of MgCl2·6H2O in six polyols"
| T/K | x1 | |||||
|---|---|---|---|---|---|---|
| 1,2-丙 二醇 | 1,3-丙 二醇 | 1,3-丁 二醇 | 一缩二 乙二醇 | 二缩三 乙二醇 | 一缩二 丙二醇 | |
| 278.15 | 0.369 6 | 0.386 1 | 0.356 8 | 0.436 1 | 0.390 8 | 0.304 2 |
| 283.15 | 0.379 6 | 0.400 2 | 0.364 7 | 0.446 4 | 0.404 5 | 0.312 8 |
| 288.15 | 0.388 5 | 0.412 4 | 0.374 1 | 0.453 2 | 0.422 1 | 0.325 7 |
| 293.15 | 0.397 1 | 0.426 2 | 0.383 9 | 0.465 8 | 0.437 9 | 0.338 6 |
| 298.15 | 0.405 3 | 0.438 1 | 0.396 9 | 0.478 9 | 0.453 6 | 0.352 3 |
| 303.15 | 0.414 2 | 0.451 1 | 0.404 1 | 0.487 7 | 0.475 6 | 0.365 9 |
| 308.15 | 0.425 8 | 0.462 1 | 0.414 9 | 0.502 2 | 0.492 5 | 0.378 5 |
| 313.15 | 0.436 2 | 0.473 9 | 0.425 8 | 0.513 1 | 0.516 8 | 0.392 1 |
| 318.15 | 0.443 5 | 0.488 3 | 0.440 9 | 0.522 8 | 0.535 7 | 0.406 8 |
| 323.15 | 0.453 4 | 0.496 3 | 0.452 5 | 0.532 1 | 0.557 8 | 0.421 4 |
| 328.15 | 0.465 7 | 0.512 9 | 0.465 2 | 0.545 3 | 0.583 9 | 0.441 3 |
Table 2
Apelblat equation parameters and average relative deviations of the solubility of MgCl2·6H2O"
| 溶剂 | A1 | B1 | C1 | RAD/% |
|---|---|---|---|---|
| 1,2-丙二醇 | -15.463 | 302.639 | 2.378 | 0.211 6 |
| 1,3-丙二醇 | 3.566 | -628.692 | -0.401 | 0.210 0 |
| 1,3-丁二醇 | -33.525 | 1 053.395 | 5.101 | 0.200 3 |
| 一缩二乙二醇 | -9.321 | 35.018 | 1.486 | 0.280 0 |
| 二缩三乙二醇 | -43.786 | 1 311.884 | 6.775 | 0.224 2 |
| 一缩二丙二醇 | -32.099 | 821.602 | 4.966 | 0.275 7 |
| 平均值 | — | — | — | 0.274 6 |
Table 3
λh equation parameters and average relative deviations of the solubility of MgCl2·6H2O"
| 溶剂 | λ | h | RAD/% |
|---|---|---|---|
| 1,2-丙二醇 | -0.494 | 3 369.724 | 0.742 9 |
| 1,3-丙二醇 | -0.429 | 2 422.762 | 0.940 4 |
| 1,3-丁二醇 | -0.417 | 3 143.828 | 0.496 6 |
| 一缩二乙二醇 | -0.592 | 2 273.479 | 0.752 6 |
| 二缩三乙二醇 | 0.007 | 1 446.312 | 0.275 2 |
| 一缩二丙二醇 | -0.217 | 3 031.885 | 0.601 6 |
| 平均值 | — | — | 0.616 2 |
Table 4
Van′t Hoff equation parameters and average relative deviations of the solubility of MgCl2·6H2O"
| 溶剂 | A2 | B2 | RAD/% |
|---|---|---|---|
| 1,2-丙二醇 | 0.501 | -417.152 | 0.303 2 |
| 1,3-丙二醇 | 0.875 | -507.305 | 0.220 3 |
| 1,3-丁二醇 | 0.724 | -491.524 | 0.544 9 |
| 一缩二乙二醇 | 0.655 | -414.808 | 0.324 9 |
| 二缩三乙二醇 | 1.717 | -743.853 | 0.702 4 |
| 一缩二丙二醇 | 1.256 | -684.774 | 0.495 9 |
| 平均值 | — | — | 0.473 2 |
Table 5
Empirical equation parameters and average relative deviations of the solubility of MgCl2·6H2O"
| 溶剂 | A3 | B3 | C3 | D3 | RAD/% |
|---|---|---|---|---|---|
| 1,2-丙二醇 | 6.822 | -5.860 | 0.019 | -1.733 | 0.189 1 |
| 1,3-丙二醇 | 1.935 | 0.000 | 0.057 | -5.881 | 0.161 2 |
| 1,3-丁二醇 | 6.200 | -4.647 | 0.013 | -1.061 | 0.213 7 |
| 一缩二乙二醇 | -2.852 | 0.000 | -0.077 | 7.878 | 0.301 0 |
| 二缩三乙二醇 | 2.284 | -3.837 | -0.002 | 0.548 | 0.223 0 |
| 一缩二丙二醇 | 1.242 | 0.000 | 0.030 | -2.897 | 0.280 3 |
| 平均值 | — | — | — | — | 0.258 8 |
Table 6
Thermodynamic parameters of MgCl2·6H2O in six polyols"
| 溶剂 | ΔHsolo / (J·mol-1) | ΔSsolo / (J·mol-1·K-1) | ΔGsolo / (J·mol-1) | ξH /% | ξTS /% |
|---|---|---|---|---|---|
| 1,2-丙二醇 | 3 468.19 | 4.16 | 2 211.90 | 73.41 | 26.59 |
| 1,3-丙二醇 | 4 217.69 | 7.30 | 2 010.81 | 65.65 | 34.35 |
| 1,3-丁二醇 | 4 086.50 | 6.03 | 2 262.17 | 69.14 | 30.86 |
| 一缩二乙二醇 | 3 448.73 | 5.42 | 1 809.73 | 67.79 | 32.21 |
| 二缩三乙二醇 | 6 184.37 | 14.30 | 1 860.00 | 58.85 | 41.15 |
| 一缩二丙二醇 | 5 693.18 | 10.52 | 2 513.52 | 64.16 | 35.84 |
| [1] | 张景红, 周启立, 张向怡. 卤水中氧化镁的提取工艺进展[J]. 无机盐工业, 2010, 42(8):7-9. |
| [2] |
HOU X H, ZHENG N P. An insight on future development approach- es of salt lake magnesium resources:From the current situation of China′s magnesium industry[J]. Acta Geologica Sinica, 2015, 88(s1):335-336.
doi: 10.1111/1755-6724.12279_12 |
| [3] | ZHANG Z, LU X, WANG T, et al. The dehydration of MgCl2·6H2O in MgCl2·6H2O-KCl-NH4Cl system[J]. Journal of Analytical and App- lied Pyrolysis, 2014, 110:248-253. |
| [4] | KIM I T, YAMABUKI K, SUMIMOTO M, et al. Characteristics of tetrahydrofuran-based electrolytes with magnesium alkoxide addi- tives for rechargeable magnesium batteries[J]. Journal of Power So- urces, 2016, 323:51-56. |
| [5] |
ZHENG T X, HU Y B, PAN F S, et al. Fabrication of corrosion-re- sistant superhydrophobic coating on magnesium alloy by one-step electrodeposition method[J]. Journal of Magnesium and Alloys, 2019, 7(2):193-202.
doi: 10.1016/j.jma.2019.05.006 |
| [6] |
HE F, LU T, FANG X, et al. Effects of strontium amount on the me- chanical strength and cell-biological performance of magnesium- strontium phosphate bioceramics for bone regeneration[J]. Materi- als Science and Engineering:C, 2020, 112.Doi: 10.1016/j.msec.2020.110892.
doi: 10.1016/j.msec.2020.110892 |
| [7] |
ZHOU H, LI J, LI J, et al. Calcium phosphate coating on biomedical WE43 magnesium alloy pretreated with a magnesium phosphate la- yer for corrosion protection[J]. Surface and Coatings Technology, 2020, 401.Doi: 10.1016/j.surfcoat.2020.126248.
doi: 10.1016/j.surfcoat.2020.126248 |
| [8] | LAN Z, LU Y C. Tailoring morphology and bulk density of magnesi- um ethoxide particles by adding n-hexane and silicone oil[J]. Par- ticuology, 2020, 53:168-174. |
| [9] | ZHANG D M, HUANG J F, GANG T M. The dehydration of magne- sium chloride by butanol distillation[J]. Journal of Central-South Institute of Mining and Metallurgy, 1988, 19(4):388-394. |
| [10] | 崔振华. 六水氯化镁在乙二醇中的溶解结晶行为研究[D]. 西宁:中国科学院青海盐湖研究所, 2014. |
| [11] | 屈俊. 六水氯化镁在乙二醇中的溶解结晶行为[J]. 化工设计通讯, 2019, 45(6):126-128,130. |
| [12] | 刘丹, 毛丽莉, 王海增. 六水氯化镁在醇类溶剂中溶解度的测定与关联[J]. 高校化学工程学报, 2018, 32(1):24-29. |
| [13] | 刘丹, 毛丽莉, 王海增. 非水体系中制备三水磷酸氢镁:中国, 107055500A[P]. 2017-08-18. |
| [14] |
LIU D, MAO L L, WANG H Z. Preparation of uniform nerberyite crystal in nonaqueous system[J]. Materials Letters, 2019, 240:169-171.
doi: 10.1016/j.matlet.2019.01.016 |
| [15] |
GAMBE J, JOUIN J, REMONDIERE F, et al. Solvent effect in the nonaqueous synjournal of ZrO2 nanoparticles under alkaline condi- tions[J]. Journal of Materials Science, 2020, 55(7):2802-2814.
doi: 10.1007/s10853-019-04137-9 |
| [16] | 杨红, 金达莱, 马照军, 等. 醇类添加剂对碳酸钙形貌及晶型的影响[J]. 浙江理工大学学报, 2007(5):549-552. |
| [17] | 靳苗苗. 二氧六环与氯化镁络合反应结晶及热分解过程研究[D]. 上海:华东理工大学, 2015. |
| [18] | 陆慕瑶. 三种苯乙酸类和一种酮类物质的固液相平衡研究[D]. 北京:北京化工大学, 2018. |
| [19] |
黄琼珠, 路贵民, 汪瑾, 等. MgCl2·6H2O热分解机理的研究[J]. 无机材料学报, 2010, 25(3):306-310.
doi: 10.3724/SP.J.1077.2010.00306 |
| [20] | 中国科学院青海盐湖研究所分析室. 卤水和盐的分析方法[M]. 北京: 科学出版社, 1988. |
| [21] | 饶念, 魏立安. 活性炭废水中钙镁离子含量测定方法研究[J]. 江西科学, 2011, 29(5):579-582. |
| [22] |
IRFAN M, WANG Y M, XU T W. Novel electrodialysis membranes with hydrophobic alkyl spacers and zwitterion structure enable high monovalent/divalent cation selectivity[J]. Chemical Engineering Journal, 2020, 383.Doi: 10.1016/j.cej.2019.123171.
doi: 10.1016/j.cej.2019.123171 |
| [23] |
LI Z L, WANG Z R, LI M Y, et al. Measurement and correlation of solubility of methyl gallate in nine pure and ethanol+n-propanol mixed solvents at temperatures within 293.15-333.15 K[J]. Jour- nal of Molecular Liquids, 2019, 293.Doi: 10.1016/j.molliq.2019.111531.
doi: 10.1016/j.molliq.2019.111531 |
| [24] |
JI W, MENG Q, LI P, et al. Measurement and correlation of the so- lubility of p-coumaric acid in nine pure and water+ethanol mixed solvents at temperatures from 293.15 to 333.15 K[J]. Journal of Chemical & Engineering Data, 2016, 61(10):3457-3465.
doi: 10.1021/acs.jced.6b00361 |
| [25] | 李涛, 沙娇, 赵瑞, 等. 双季戊四醇在3种混合溶剂中的固-液相平衡[J]. 化工学报, 2020, 71(1):245-253. |
| [26] | APELBLAT A, MANZUROL E. Solubilities ofo-acetylsalicylic,4- aminosalicylic,3,5-dinitrosalicylic,andp-toluic acid,and magne- sium-DL-aspartate in water from T=(278 to 348) K[J]. The Jo- urnal of Chemical Thermodynamics, 1999, 31(1):85-91. |
| [27] | 陈丽芳, 张勤勤, 林田, 等. 硼酸在氯化钙溶液中溶解度的测定与关联[J]. 无机盐工业, 2019, 51(5):70-73. |
| [28] |
WANG M, LIU Y, DU S, et al. Determining the solubility and un- derstanding the solid-liquid equilibrium behavior of cyhalothric acid in eleven pure solvents[J]. Journal of Molecular Liquids, 2020, 300.Doi: 10.1016/j.molliq.2019.112365.
doi: 10.1016/j.molliq.2019.112365 |
| [29] |
BUCHOWSKI H, KSIAZCZAK A, PIETRZYK S. Solvent activity along a saturation line and solubility of hydrogen-bonding solids[J]. The Journal of Physical Chemistry, 1980, 84(9):975-979.
doi: 10.1021/j100446a008 |
| [30] |
WU Y F, WU Y C, ZHANG X L. The dissolution behavior and ap- parent thermodynamic analysis of propacetamol hydrochloride in pure and mixed solvents[J]. The Journal of Chemical Thermodyna- mics, 2020, 143.Doi: 10.1016/j.jct.2019.106018.
doi: 10.1016/j.jct.2019.106018 |
| [31] |
LI D, CAO D L, CHEN L Z, et al. Solubility of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate in(formic acid,water) and their binary solvents from 298.15 K to 333.15 K at 101.1 kPa[J]. The Journal of Chemical Thermodynamics, 2019, 128:10-18.
doi: 10.1016/j.jct.2018.07.030 |
| [32] | 张莉媛, 王刚, 齐美玲, 等. 表面活性剂对光卤石中氯化钾结晶介稳区和诱导期的影响[J]. 无机盐工业, 2020, 52(6):46-49. |
| [33] |
XIAO M, LIU H, WANG J, et al. An experimental and modeling study of physical N2O solubility in 2-(ethylamino) ethanol[J]. The Journal of Chemical Thermodynamics, 2019, 138:34-42.
doi: 10.1016/j.jct.2019.06.008 |
| [34] | WONGKAEW K, FULAJTAROVA K, HRONEC M, et al. Measu- rement of the solubility of the salt of 2-mercaptobenzothiazole with cyclohexylamine and tert-butylamine in various solvents at low temperatures:Models and thermodynamic parameters[J]. Fluid Ph- ase Equilibria, 2017, 434:141-151. |
| [35] |
GUO H, CAO D, LIU Y, et al. Determination and correlation of so- lubility of N-methyl-3,4,5-trinitropyrazole(MTNP) in ten pure solvents from 283.15 K to 323.15 K[J]. Fluid Phase Equilibria, 2017, 444:13-20.
doi: 10.1016/j.fluid.2017.04.008 |
| [36] |
LI R, YIN X, ZHANG J, et al. Improving the solubility of temozolo- mide by cosolvent and its correlation with the Jouyban-Acree and CNIBS/RK models[J]. The Journal of Chemical Thermodynamics, 2019, 139.Doi: 10.1016/j.jct.2019.07.017.
doi: 10.1016/j.jct.2019.07.017 |
| [37] | 谢永, 蒋奇齐, 王红艳, 等. 对二甲氨基苯甲醛在不同的溶剂中溶解度测定与关联[J]. 高校化学工程学报, 2017, 31(2):284-290. |
| [38] |
ZHANG Y, GUO F, CUI Q, et al. Measurement and correlation of the solubility of vanillic acid in eight pure and water+ethanol mixed solvents at temperatures from(293.15 to 323.15) K[J]. Journal of Chemical & Engineering Data, 2016, 61(1):420-429.
doi: 10.1021/acs.jced.5b00619 |
| [1] | FENG Xia, YU Xuefeng, YAO Zhihao, LUO Jun, REN Siying, ZHAO Zhixing, YU Xudong. Study on phase equilibria of aqueous ternary system of Na+(Mg2+),Ca2+∥Cl--H2O at 278.2 K [J]. Inorganic Chemicals Industry, 2024, 56(1): 47-52. |
| [2] | DENG Wenqing,ZHU Jing,FAN Xiaojuan,CHEN Yan,LI Tianxiang. Phase equilibria of ternary system of KH2PO4-(NH2)2CO-H2O at 313.15 K [J]. Inorganic Chemicals Industry, 2023, 55(1): 100-105. |
| [3] | LI Yan,WANG Yansong,CHENG Huaigang,KANG Jin,LI Enze,LÜ Hongzhou,LIU Qian. Rapid quality inspection of high-purity lithium carbonate based on determination of magnesium by water-soluble probe method [J]. Inorganic Chemicals Industry, 2023, 55(1): 87-92. |
| [4] | CHEN Shuai,YANG Bo,CHEN Niancu,LUO Jun,REN Siying,ZENG Ying,YU Xudong. Study on phase equilibria of ternary system NH4Cl+MgCl2+H2O at 323.2 K [J]. Inorganic Chemicals Industry, 2022, 54(4): 100-103. |
| [5] | MA Yujun,WANG Xiao,LI Shuya,ZHANG Fukang,LI Juan. Phase equilibria of quaternary system of NaCl+NaBO2+Na2CO3+H2O at 298.15 K [J]. Inorganic Chemicals Industry, 2022, 54(10): 42-46. |
| [6] | ZHANG Huanhuan,CHENG Zhuo,TANG Xiuhua,ZHANG Fengzhen. Determination and correlation of solubility of potassium dihydrogen phosphate in acetonitrile-water solvent [J]. Inorganic Chemicals Industry, 2022, 54(1): 83-85. |
| [7] | Wang Bin,Deng Xiaochuan,Shi Yifei,Dong Chaochao,Fan Faying,Zhu Chaoliang,Fan Jie. Online determination of the solubility of lithium carbonate in water and NaCl-KCl solution system [J]. Inorganic Chemicals Industry, 2021, 53(7): 73-79. |
| [8] | Yang Jiamin,Zhu Jing,Hu Xue,Wu Qiang,Li Tianxiang. Determination and correlation for solid-liquid phase equilibrium of ternary KCl-NH4Cl-H2O and KH2PO4-NH4H2PO4-H2O systems at 283.15 K [J]. Inorganic Chemicals Industry, 2021, 53(1): 30-35. |
| [9] | Liu Juexin,Zheng Chenggang,Ye Shichao. Determination and application of thermodynamic data of potassium dihydrogen phosphate crystal [J]. Inorganic Chemicals Industry, 2021, 53(1): 62-64. |
| [10] | Zhang Liyuan,Wang Gang,Qi Meiling,Xie Yulong. Effect of surfactants on metastable zone and induction period of KCl crystals in carnallite [J]. Inorganic Chemicals Industry, 2020, 52(6): 46-49. |
| [11] | Wang Jukui,Dong Xingfeng,Zhao Dong,Wang Shiqiang,Guo Yafei,Deng Tianlong. Solid-liquid phase equilibria in quaternary system lithium borate-potassium borate-magnesium borate-water at 308.15 K [J]. Inorganic Chemicals Industry, 2020, 52(5): 27-30. |
| [12] | Qi Meiling,Wang Gang,Zhang Liyuan,Xie Yulong. Determination of KCl solubility,interfacial region and induction period in carnallite [J]. Inorganic Chemicals Industry, 2020, 52(5): 45-49. |
| [13] | Wang Yubin,Wang Wangbo,Zhu Xinfeng,Xun Jingwen,Dang Weiben. Effect of different magnesium salts on solubility behavior of calcium carbonate under magnetization [J]. Inorganic Chemicals Industry, 2020, 52(3): 35-38. |
| [14] | Wu Qiang,Hu Xue,Zhu Jing,Yang Jiamin,Li Tianxiang. Phase equilibrium of KH2PO4-KCl-H2O and NH4H2PO4-NH4Cl-H2O ternary system at 283.15 K [J]. Inorganic Chemicals Industry, 2020, 52(11): 24-28. |
| [15] | Cao Peng. Effect of acid soluble main reverse on solubility of solid phase of titanium white production by sulfuric acid process [J]. Inorganic Chemicals Industry, 2019, 51(9): 54-56. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||