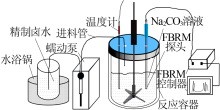
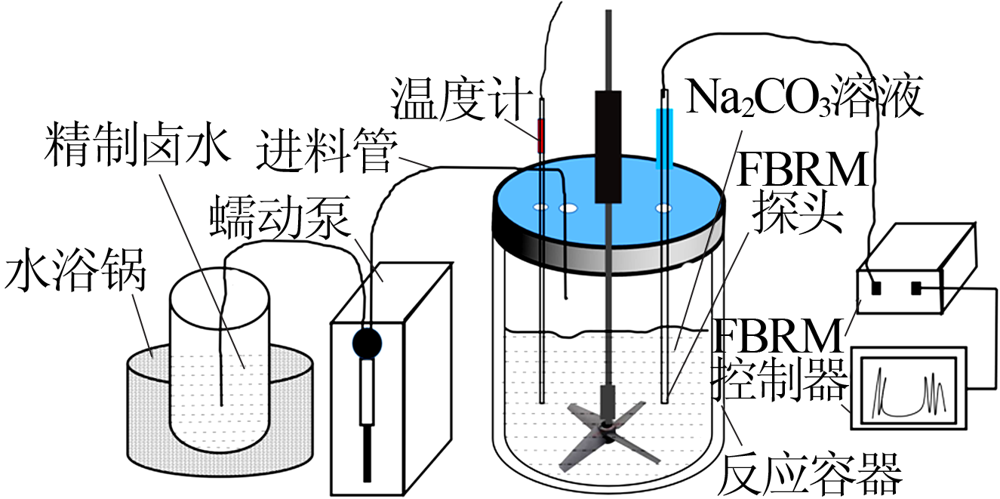
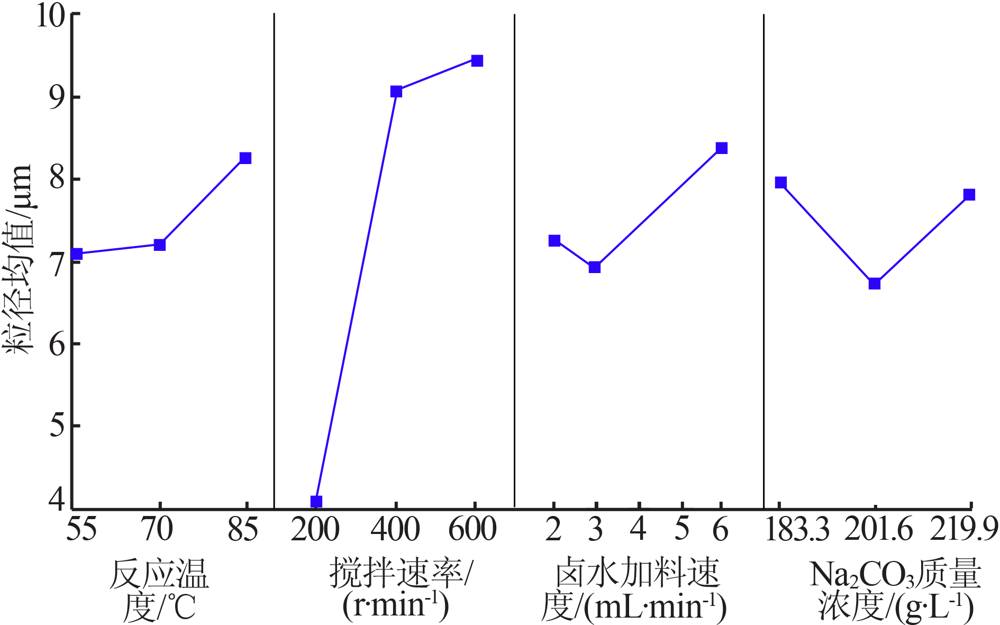
Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (8): 60-65.doi: 10.19964/j.issn.1006-4990.2020-0527
• Research & Development • Previous Articles Next Articles
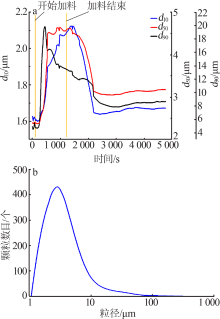
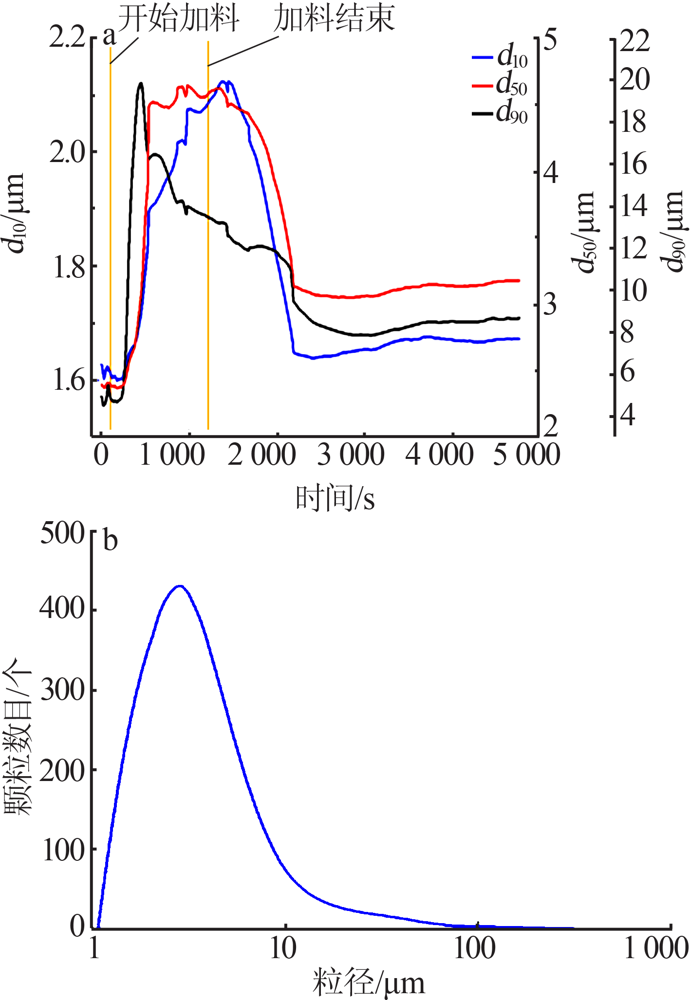
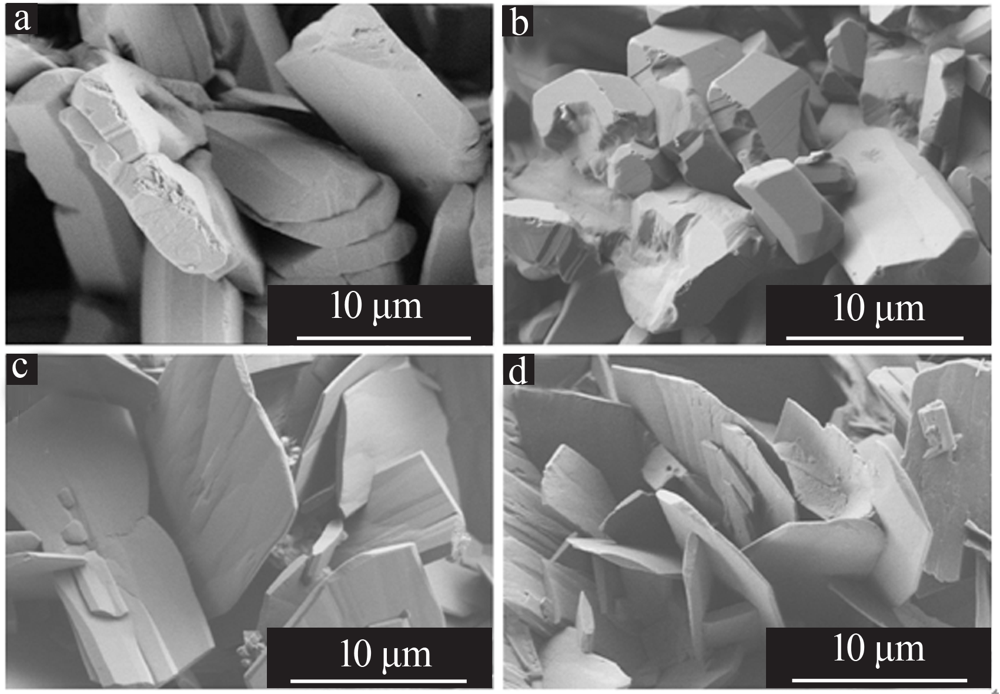
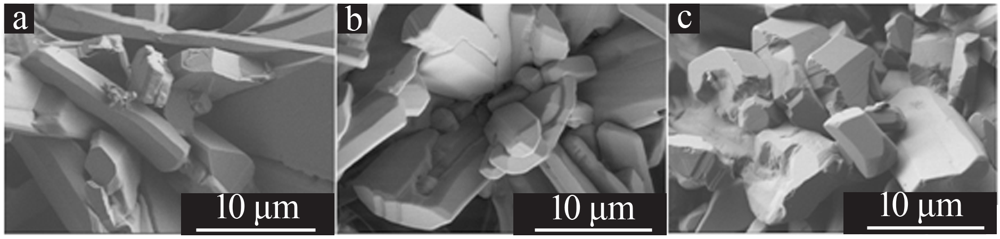
Optimizing preparation process of lithium carbonate reaction crystallization by Taguchi experimental design method
Wang Bin1,2,3,4( ),Deng Xiaochuan1,2(
),Deng Xiaochuan1,2( ),Shi Yifei1,2,Dong Chaochao1,2,3,Fan Faying1,2,Zhu Chaoliang1,2,Fan Jie1,2,Ma Wanxia1,2,Zuo Fangtao1,2
),Shi Yifei1,2,Dong Chaochao1,2,3,Fan Faying1,2,Zhu Chaoliang1,2,Fan Jie1,2,Ma Wanxia1,2,Zuo Fangtao1,2
- 1. Key Laboratory of Comprehensive and Highly Efficient Utilization of Salt Lake Resources,Qinghai Institute of Salt Lakes,Chinese Academy of Sciences,Xining 810008,China
2. Qiughai Engineering and Technology Research Center of Comprehensive Utilization of Salt Lake Resources
3. University of Chinese Academy of Sciences
4. Yinchuan Branch of State Power Science & Research Institute Co.,Ltd.