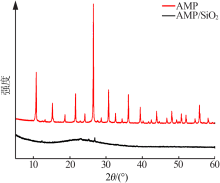
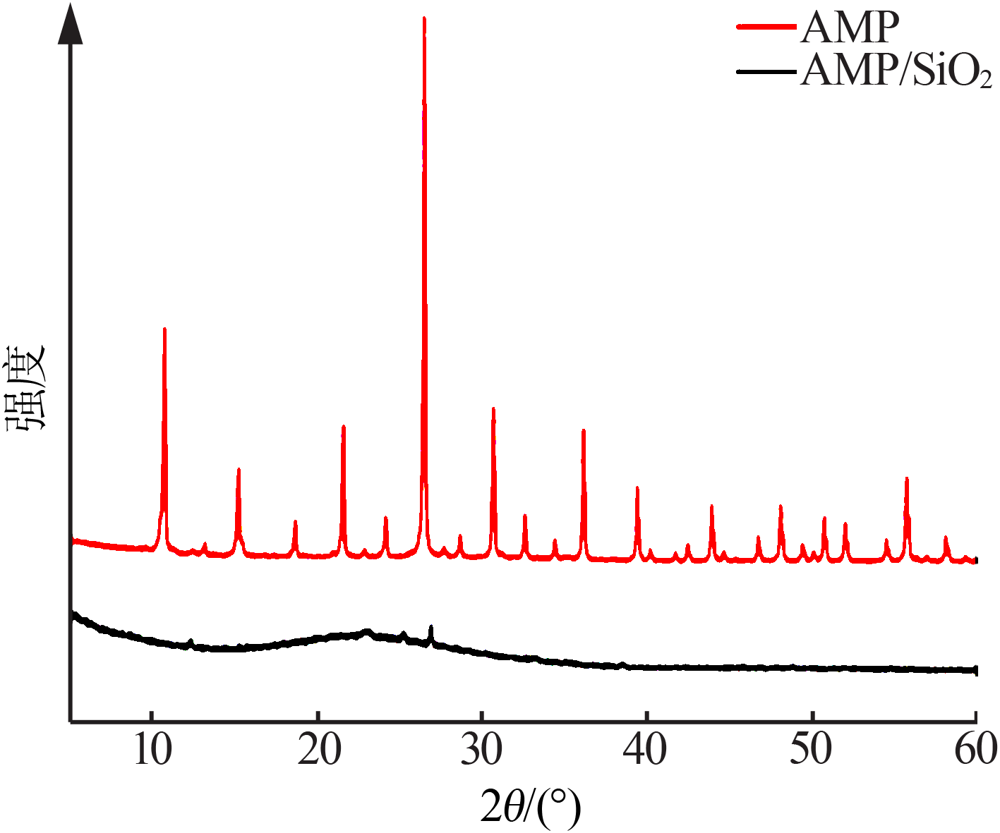
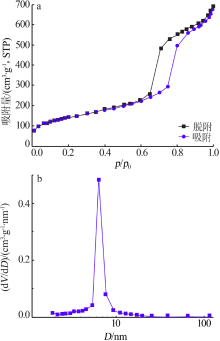
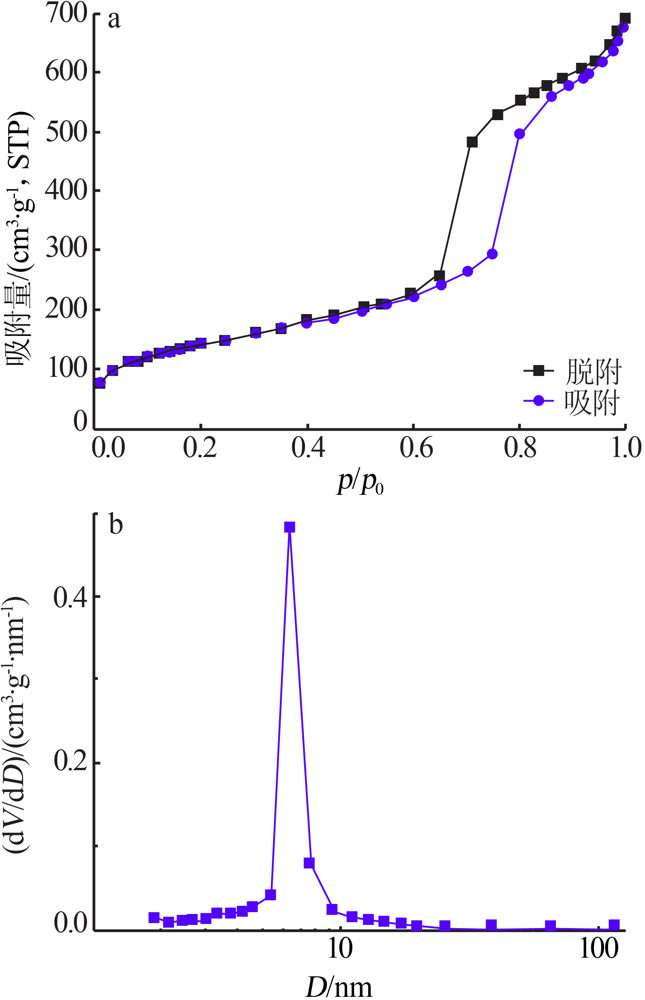
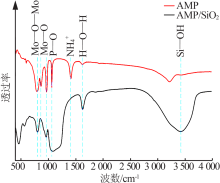
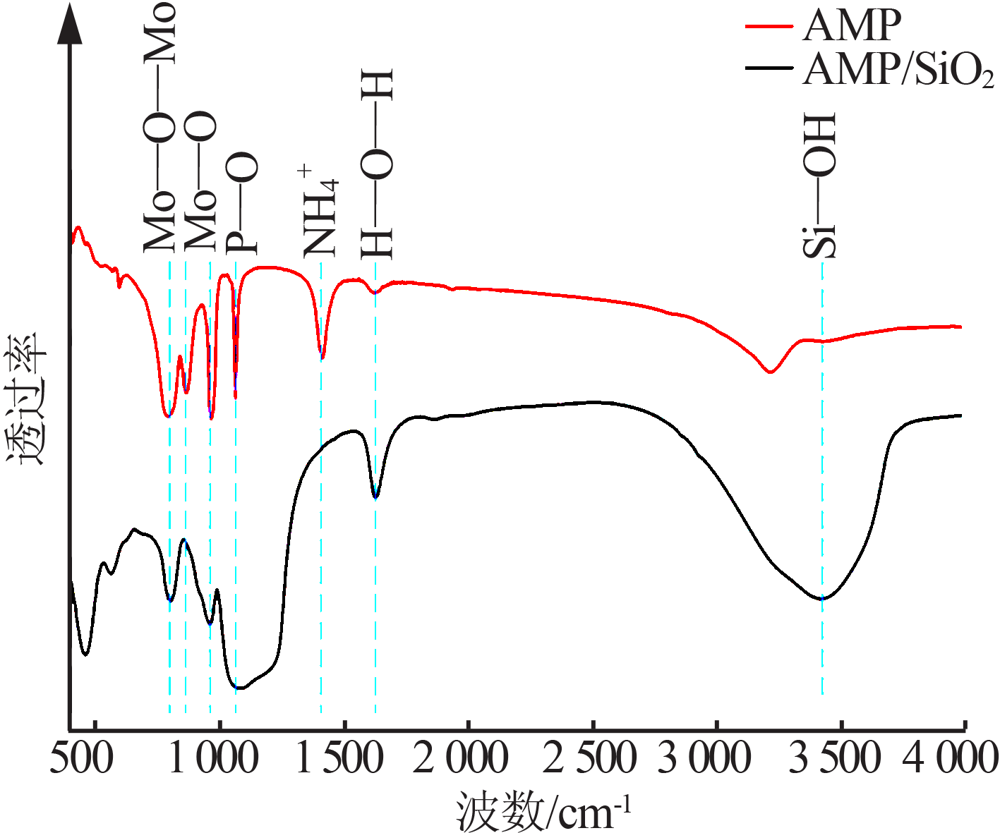
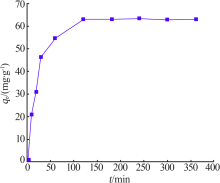
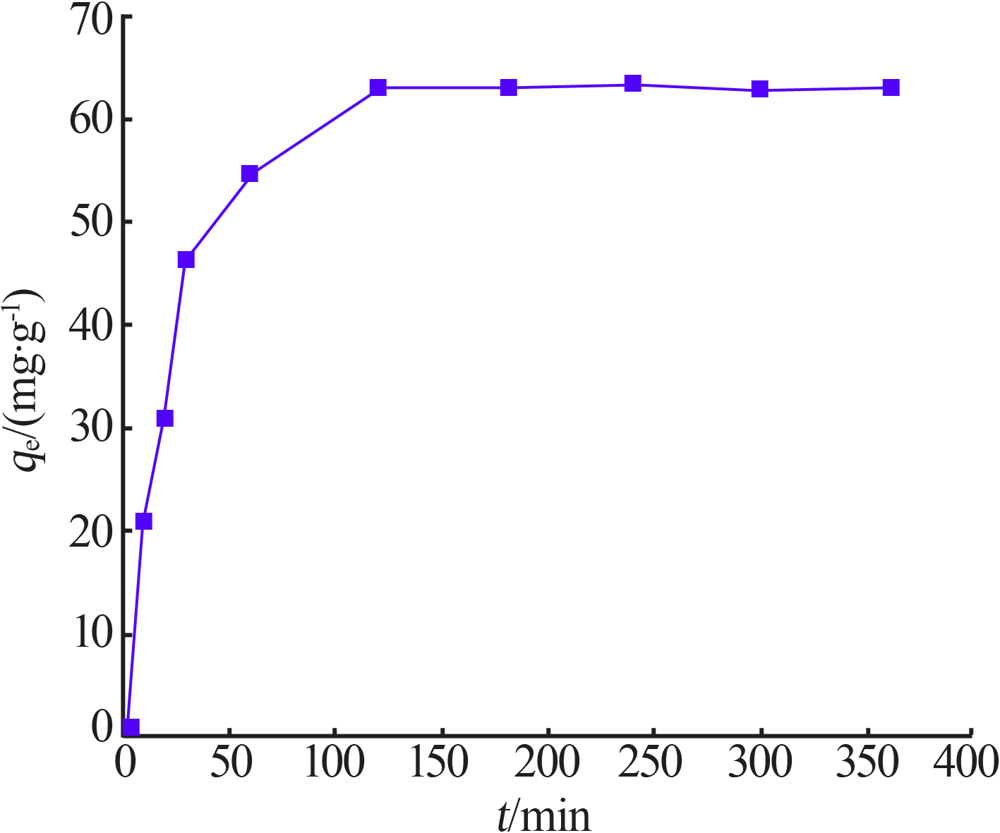
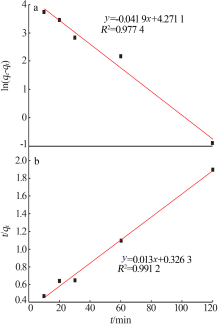
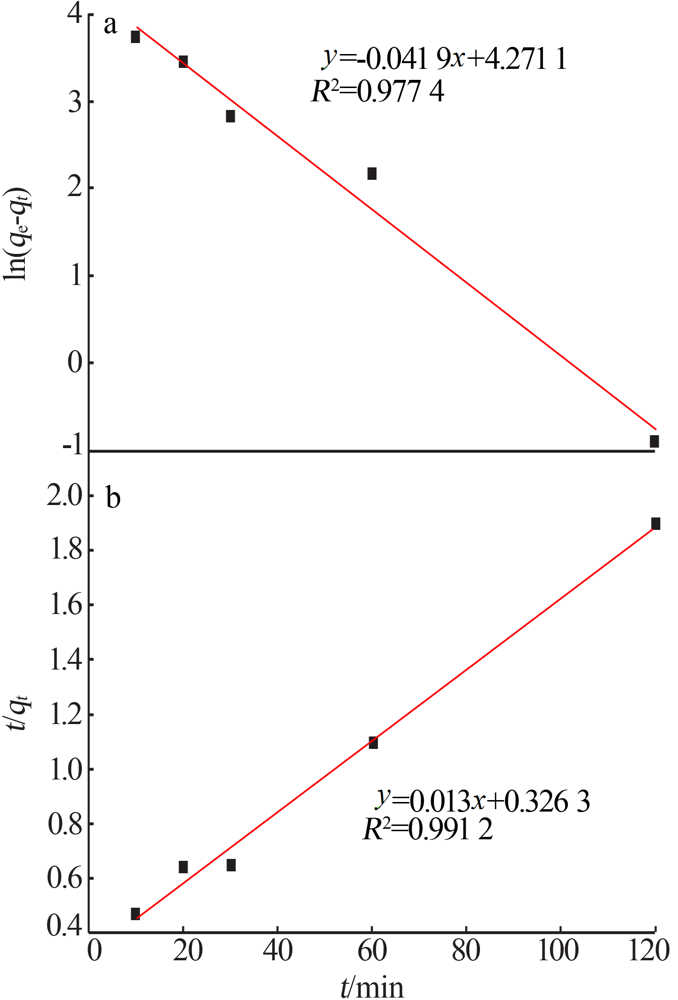
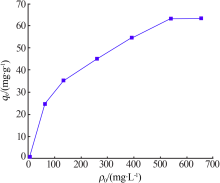
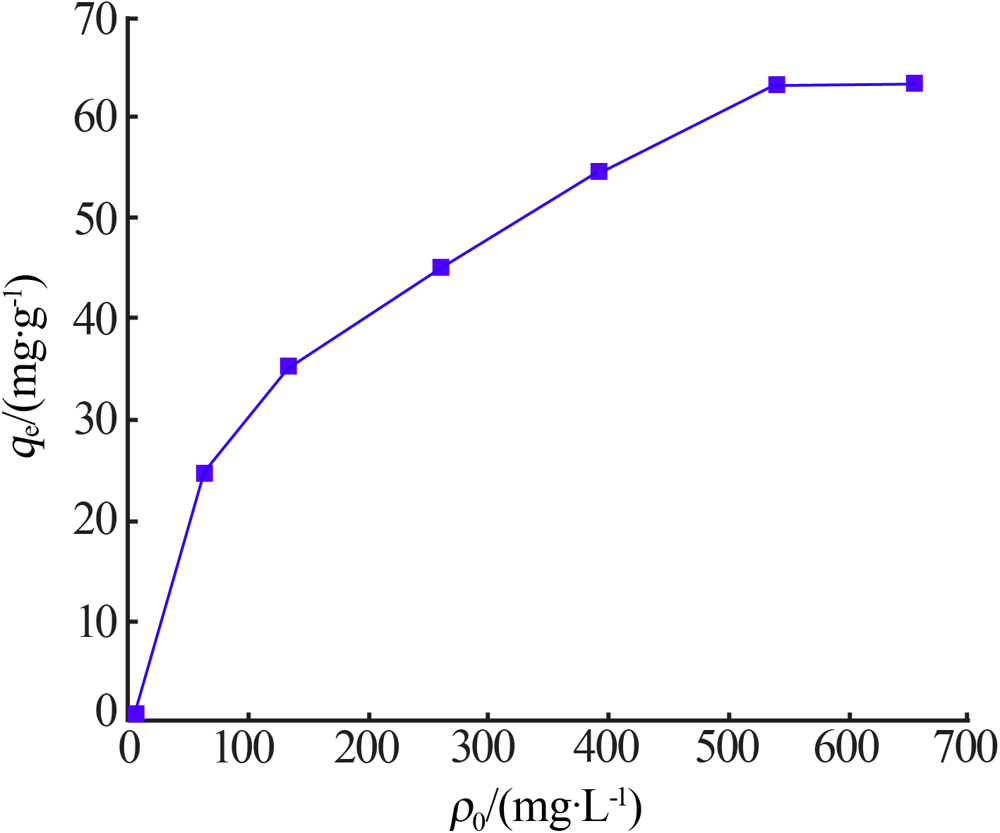
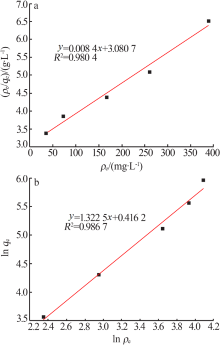
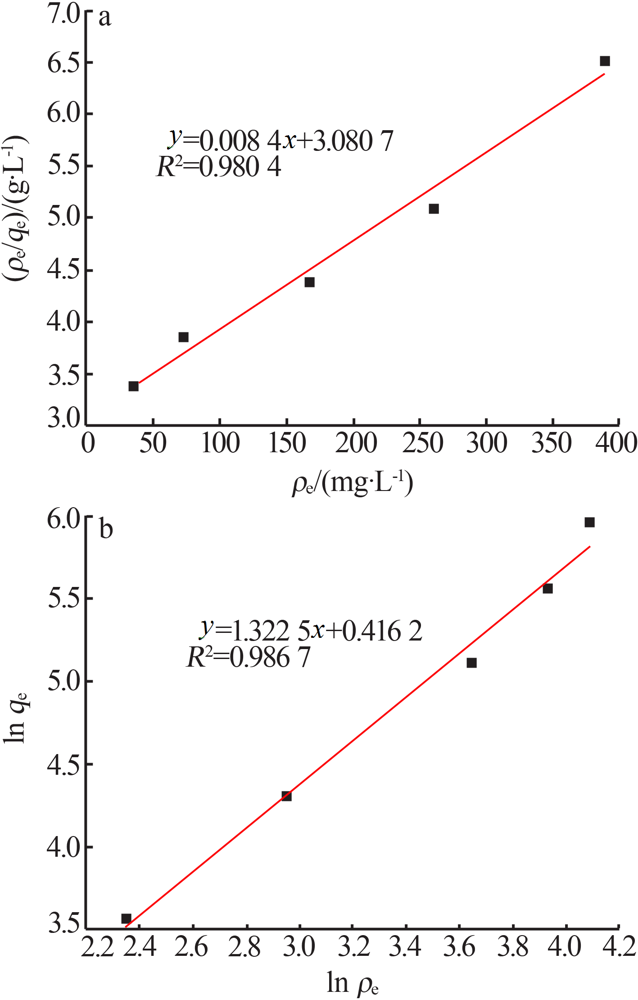
Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (6): 134-139.doi: 10.19964/j.issn.1006-4990.2020-0436
• Research & Development • Previous Articles Next Articles
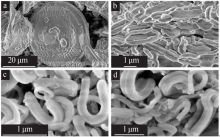
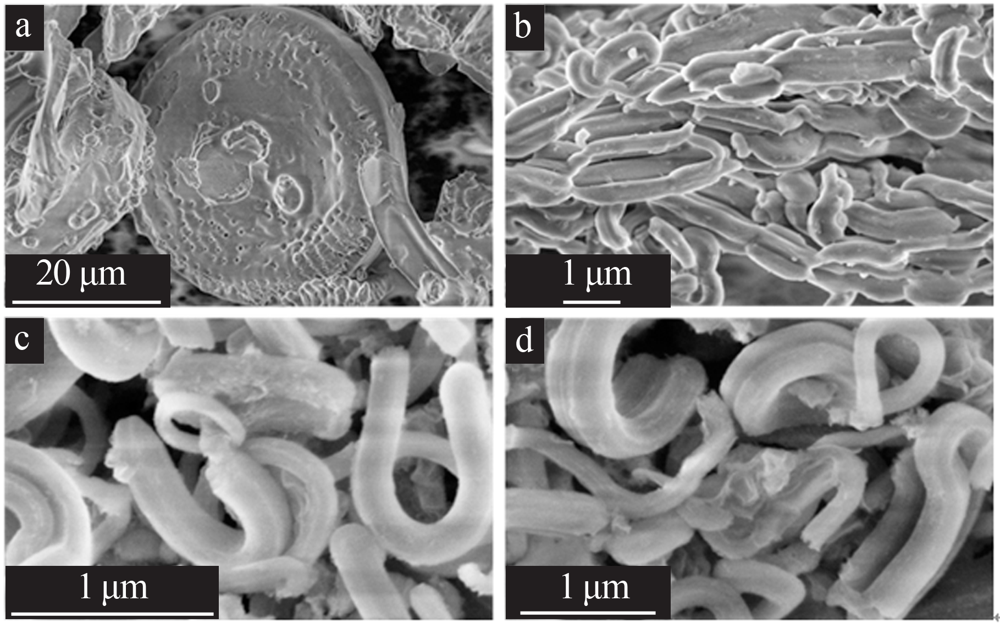
Preparation of cesium adsorbent from diatomite and its adsorption properties
Dong Chaochao1,2,3( ),Deng Xiaochuan1,2,Wang Bin1,2,3,Fan Faying1,2,Zhu Chaoliang1,2,Fan Jie1,2,Qing Binju1,2(
),Deng Xiaochuan1,2,Wang Bin1,2,3,Fan Faying1,2,Zhu Chaoliang1,2,Fan Jie1,2,Qing Binju1,2( )
)
- 1. Key Laboratory of Comprehensive and Highly Efficient Utilization of Salt Lake Resources,Qinghai Instituteof Salt Lakes,Chinese Academy of Sciences,Xining 810008,China
2. Qinghai Engineering and Technology Research Center of Comprehensive Utilization of Salt Lake Resources
3. University of Chinese Academy of Sciences
-
Received:2020-09-25Online:2021-06-10Published:2021-07-08 -
Contact:Qing Binju E-mail:dongchaochao18@mails.ucas.edu.cn;qbj@isl.ac.cn
CLC Number:
Cite this article
Dong Chaochao,Deng Xiaochuan,Wang Bin,Fan Faying,Zhu Chaoliang,Fan Jie,Qing Binju. Preparation of cesium adsorbent from diatomite and its adsorption properties[J]. Inorganic Chemicals Industry, 2021, 53(6): 134-139.
share this article
| [1] | Hamlyn R, Mahapatra M, Orozco I, et al. Morphology and chemical behavior of model CsOx/Cu2O/Cu(111) nanocatalysts for methanol synjournal:Reaction with CO2 and H2[J]. Journal of Chemical Phy-sics, 2020,152(4):1-7. |
| [2] | Parida B, Yoon S, Jeong S M, et al. Recent progress on cesium lead/ tin halide-based inorganic perovskites for stable and efficient solar cells:A review[J]. Solar Energy Materials and Solar Cells, 2020,204:1-29. |
| [3] | Saleem M I, Yang S Y, Sulaman M, et al. All-solution-processed UV-IR broadband trilayer photodetectors with CsPbBr3 colloidal nanocrystals as carriers-extracting layer[J]. Nanotechnology, 2020,31(16):1-9. |
| [4] | 宝阿敏, 钱志强, 郑红, 等. 铷、铯的分离提取方法及其研究进展[J]. 应用化工, 2017,46(7):1377-1382. |
| [5] | 张晶晶, 周蕾, 刘萌, 等. 放射性废水中铯的去除方法研究进展[J]. 化学通报, 2019,82(1):12-17. |
| [6] | 刘泽宇, 王斌, 杨晋生, 等. t-BAMBP/磺化煤油体系萃取溶液中微量铯[J]. 盐湖研究, 2019,27(3):75-80. |
| [7] | 黄建成, 丁冬, 李玉婷, 等. 生物质碳气凝胶/MnO2复合电极对Rb+、Cs+的电吸附行为[J]. 无机盐工业, 2019,51(10):72-76. |
| [8] | 陈侠, 李颖. 离子交换法富集铷、铯研究进展[J]. 无机盐工业, 2019,51(4):6-9. |
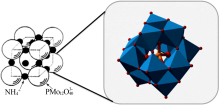
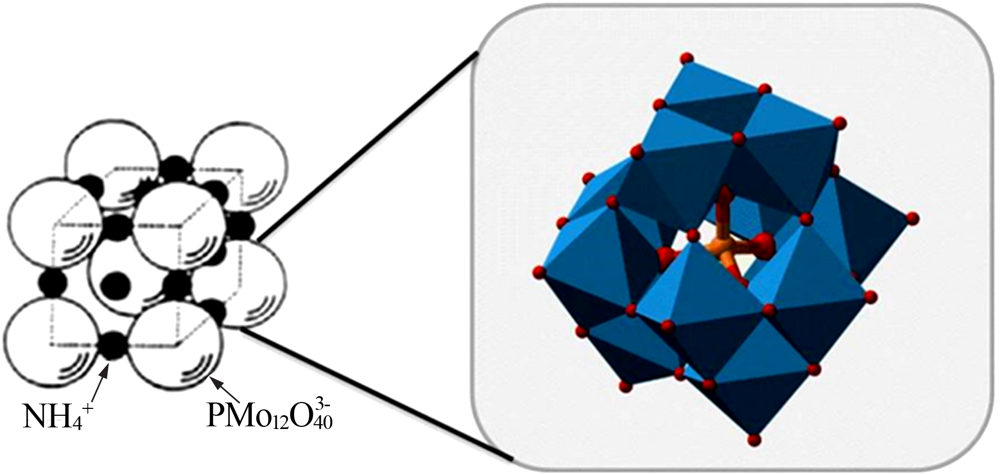
| [9] | 李颖, 陈侠, 苗淑兰, 等. 磷钨酸铵复合材料对铷铯吸附性能的研究[J]. 无机盐工业, 2019,51(6):34-37. |
| [10] | Heo Y S, Ryu J M, Park S M, et al. Structural basis for inhibition of protein tyrosine phosphatases by Keggin compounds phosphomoly-bdate and phosphotungstate[J]. Experimental and Molecular Me-dicine, 2002,34(3):211-223. |
| [11] |
Smit J, Van R. Ammonium salts of the heteropolyacids as cation ex-changers[J]. Nature, 1958,181(4622):1530-1531.
doi: 10.1038/1811530a0 |
| [12] | Wang J L, Zhuang S T. Removal of cesium ions from aqueous solu-tions using various separation technologies[J]. Reviews in Enviro-nmental Science and Bio-Technology, 2019,18(2):231-269. |
| [13] | Ye X S, Wu Z J, Li W, et al. Rubidium and cesium ion adsorption by an ammonium molybdophosphate-calcium alginate composite adsorbent[J]. Colloids and Surfaces A-Physicochemical and En-gineering Aspects, 2009,342(1/2/3):76-83. |
| [14] | Chen S Q, Hu J Y, Shi J, et al. Composite hydrogel particles enca-psulated ammonium molybdophosphate for efficiently cesium sele-ctive removal and enrichment from wastewater[J]. Journal of Ha-zardous Materials, 2019,371:694-704. |
| [15] |
Deng H, Li Y X, Huang Y, et al. An efficient composite ion exchan-ger of silica matrix impregnated with ammonium molybdophosphate for cesium uptake from aqueous solution[J]. Chemical Engineering Journal, 2016,286:25-35.
doi: 10.1016/j.cej.2015.10.040 |
| [16] | 邵艳秋, 张宇婷, 姜振双, 等. 水解剂氟化铵对Fe-SBA-15结构及催化性能的影响[J]. 无机盐工业, 2019,51(9):97-101. |
| [17] | 王冬华. 氧化锌/SBA-15复合材料制备及其光致发光性质[J]. 无机盐工业, 2018,50(4):27-29,42. |
| [18] | Selim A Q, Mohamed E A, Mobarak M, et al. Cr(Ⅵ) uptake by a composite of processed diatomite with MCM-41:Isotherm,kinetic and thermodynamic studies[J]. Microporous and Mesoporous Ma-terials, 2018,260:84-92. |
| [19] |
Sun C Y, Zhang F, Li S F, et al. Synjournal of SBA-15 encapsulated ammonium molybdophosphate using Qaidam natural clay and its use in cesium ion adsorption[J]. RSC Advances, 2015,5(45):35453-35460.
doi: 10.1039/C5RA02398J |
| [20] |
Park Y, Shin W S, Choi S J. Ammonium salt of heteropoly acid im-mobilized on mesoporous silica(SBA-15):An efficient ion exch-anger for cesium ion[J]. Chemical Engineering Journal, 2013,220:204-213.
doi: 10.1016/j.cej.2013.01.027 |
| [21] | Dan H, Xian Q, Chen L, et al. One-step direct synjournal of mesopo-rous AMP/SBA-15 using PMA as acid media and its use in cesium ion removal[J]. Journal of Nuclear Materials, 2019,527:1-8. |
| [22] | 刘澜. 改性稻秆吸附剂表征及处理亚甲基蓝溶液的吸附性能研究[D]. 重庆:重庆大学, 2011. |
| [23] |
Murthy G S, Sivaiah M V, Kumar S S, et al. Adsorption of cesium on a composite inorganic exchanger zirconium phosphate-ammoni-um molybdophosphate[J]. Journal of Radioanalytical and Nuclear Chemistry, 2004,260:109-114.
doi: 10.1023/B:JRNC.0000027068.15669.ad |
| [24] | Park Y, Lee Y C, Shin W S, et al. Removal of cobalt,strontium and cesium from radioactive laundry wastewater by ammonium molyb-dophosphate-polyacrylonitrile(AMP-PAN)[J]. Chemical Engin-eering Journal, 2010,162:685-695. |
| [25] | Chakravarty R, Ram R, Pillai K T, et al. Ammonium molybdophos-phate impregnated alumina microspheres as a new generation sor-bent for chromatographic137Cs/137mBa generator[J]. Journal of Ch-romatography A, 2012,1220:82-91. |
| [26] | Dan H, Xian Q, Chen L. Fabrication of AMP/SBA-15 with various morphologies for cesium removal from aqueous solution[J]. Jour-nal of Sol-Gel Science and Technology, 2019,91:165-177. |
| [1] | DI Lu, WANG Weiguo, CHEN Juexian, WU Chuanshu. Study on preparation of transition metal-supported Silicalite-1 zeolite catalyst and its catalytic performance for furfural hydrogenation [J]. Inorganic Chemicals Industry, 2024, 56(4): 125-132. |
| [2] | XU Mengyao, ZHANG Xin, HE Kunpeng, HE Jian, JIANG Wei. Preparation of yttrium oxide and zirconium phosphate adsorbents from zirconium-yttrium waste and evaluation of their performance [J]. Inorganic Chemicals Industry, 2024, 56(3): 116-124. |
| [3] | LI Qiaoyun, HUANG Xiuxing, WEI Wenye, CHEN Zhen. Study on adsorption of methylene blue by activated carbon with acid/alkali synergistically modified fly ash [J]. Inorganic Chemicals Industry, 2024, 56(3): 131-136. |
| [4] | WANG Jianping, XUE Xujin, XUE Fengfeng. Study on new process of preparing high-purity aluminium fluoride from fluorosilicic acid [J]. Inorganic Chemicals Industry, 2024, 56(3): 86-90. |
| [5] | LI Yang, LOU Feijian, SUI Xin, LI Keyan, LIU Fei, GUO Xinwen. Preparation of amine-functionalized fumed SiO2 materials and their performance for CO2 adsorption [J]. Inorganic Chemicals Industry, 2024, 56(2): 38-43. |
| [6] | ZHANG Li, ZHANG Dan, PAN Hongyan, DONG Yonggang, LI Wenfei, QIN Hong. Study on preparation of low ash activated carbon by phosphoric acid method [J]. Inorganic Chemicals Industry, 2024, 56(2): 95-103. |
| [7] | YAN Zhen, QIU Zhaofu, JIN Xibiao, WANG Yuan, LIU Chang, YANG Ji. Study on 4A molecular sieve loaded with Ce and γ-Fe2O3 for removal of Sb(Ⅲ) and Sb(Ⅴ) in water [J]. Inorganic Chemicals Industry, 2024, 56(1): 81-89. |
| [8] | ZHOU Shiqi, WANG Tao, JING Fangli, LUO Shizhong. Study on performance of magnesium nitrate-modified carbon molecular sieve for separation of nitrogen/methane [J]. Inorganic Chemicals Industry, 2023, 55(9): 75-80. |
| [9] | LI Chao, WANG Liping, DAI Yin, GAO Guimei, ZHANG Yunfeng, HONG Yu, XU Lijun, CUI Yongjie. Study on alkali fusion hydrothermal synthesis of 13X zeolite from high silicon tailings and its adsorption on lead,copper and zinc ions [J]. Inorganic Chemicals Industry, 2023, 55(9): 88-93. |
| [10] | LUO Wenbo, LI Heng, LÜ Jun, YANG Linguang, ZHAO Xingfan, LONG Xiao. Study on recovery of silicon and aluminum from industrial silicon slag [J]. Inorganic Chemicals Industry, 2023, 55(9): 94-99. |
| [11] | WANG Yingnan, SHENG Linlin, HUANG Juan, HUANG Zhanbin. Study on adsorption performance of lead from water by coal-fired slag [J]. Inorganic Chemicals Industry, 2023, 55(8): 109-115. |
| [12] | TANG Yifu, CAO Changchun, LÜ Peng. Study on adsorption of cadmium-humic acid by hydroxyiron oxide [J]. Inorganic Chemicals Industry, 2023, 55(8): 124-131. |
| [13] | HAN Hongjing, ZHANG Jingze, LAMAO Zhuoma, HAN Jizhe, WU Yongmin, TANG Weiping. Preparation of Al-Co co-doped lithium manganese oxide and its adsorption performance of lithium [J]. Inorganic Chemicals Industry, 2023, 55(7): 38-44. |
| [14] | FAN Fangfang, TONG Zhongkai, ZUO Weiyuan. Study on adsorption of tetracycline from wastewater by calcium modified peanut shell biochar [J]. Inorganic Chemicals Industry, 2023, 55(6): 109-115. |
| [15] | TIAN Yuling, CHENG Yang, HAN Rong, ZHOU Mei, WANG Chengjie, GE Qiangru. Effect of CaO on heavy metals stability and adsorption properties of sludge-derived biochar [J]. Inorganic Chemicals Industry, 2023, 55(6): 124-129. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||