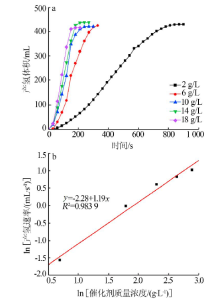
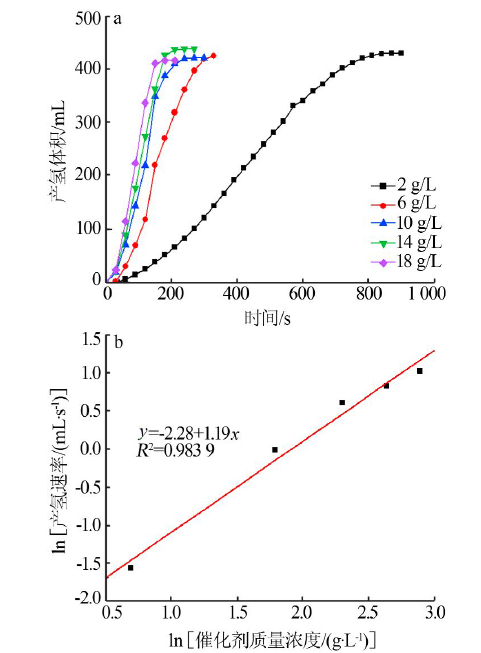
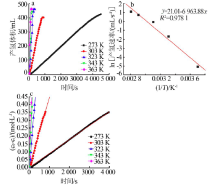
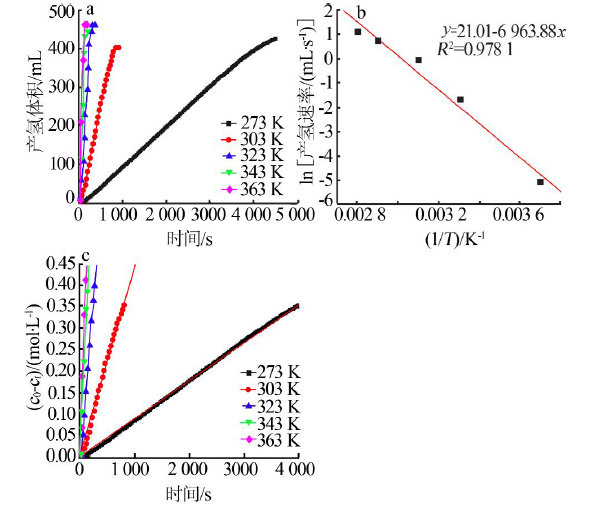
Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (1): 102-106.doi: 10.11962/1006-4990.2020-0061
Previous Articles Next Articles
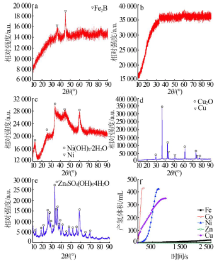
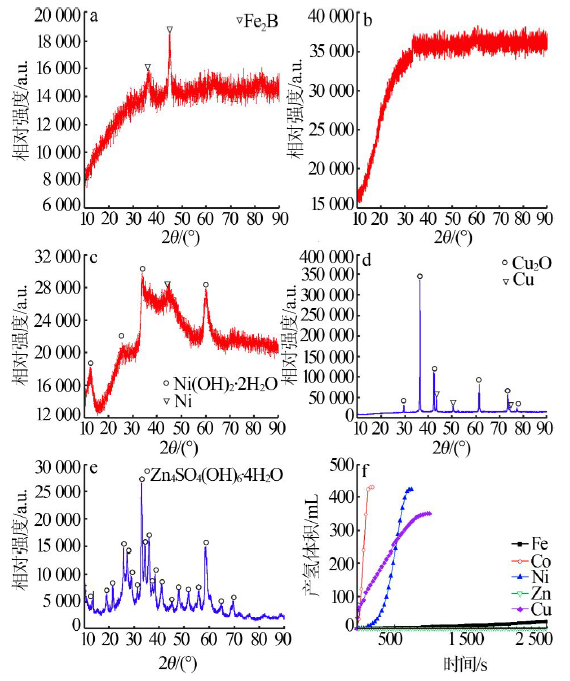
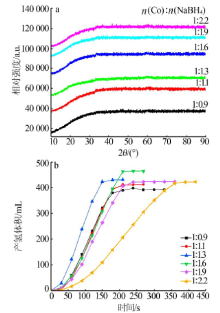
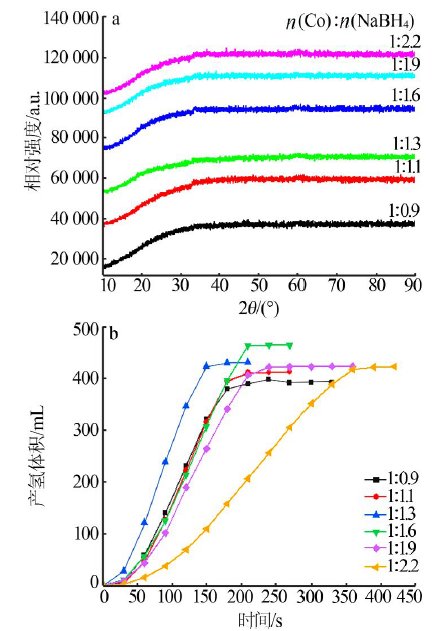
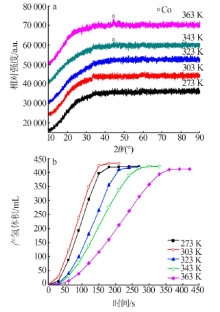
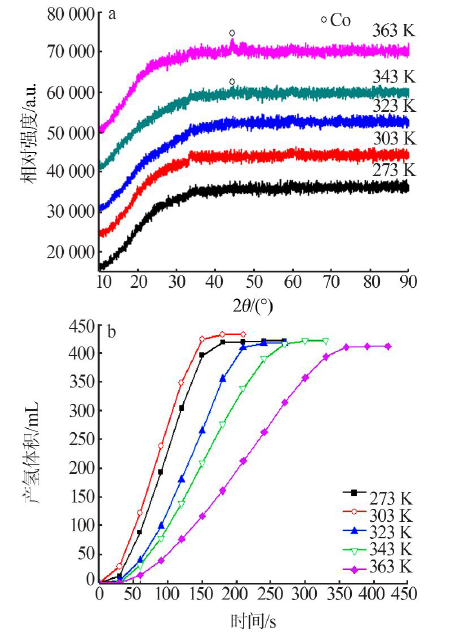
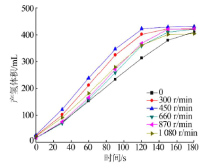
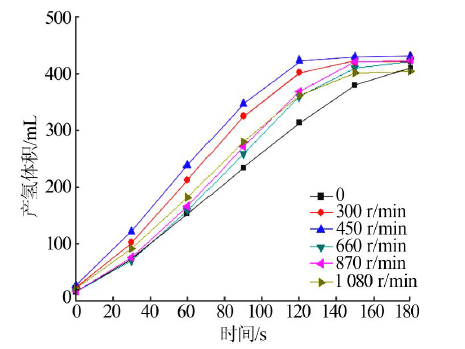
Performance of Fe,Co,Ni,Cu and Zn catalysts for hydrogen generation from catalytic hydrolysis of ammonia borane
Sun Haijie1( ),Mei Yangyang1,Chen Zhihao2,Chen Lingxia1,Zhang Qiaoyu1,Liu Xingai1
),Mei Yangyang1,Chen Zhihao2,Chen Lingxia1,Zhang Qiaoyu1,Liu Xingai1
- 1. School of Chemistry and Chemical Engineering,Zhengzhou University,Zhengzhou 450044,China
2. Zhengzhou Tobacco Research Institute of CNTC
-
Received:2020-07-13Online:2021-01-10Published:2021-01-08
CLC Number:
Cite this article
Sun Haijie,Mei Yangyang,Chen Zhihao,Chen Lingxia,Zhang Qiaoyu,Liu Xingai. Performance of Fe,Co,Ni,Cu and Zn catalysts for hydrogen generation from catalytic hydrolysis of ammonia borane[J]. Inorganic Chemicals Industry, 2021, 53(1): 102-106.
share this article
| [1] |
李慧珍, 王芃远, 陈学年. 氨硼烷:一种高性能化学储氢材料[J]. 科学通报, 2014,59(19):1823-1837.
doi: 10.1360/972013-1221 |
| [2] | 孙海杰, 陈凌霞, 张玉凤, 等. 钴-硼/二氧化锆催化剂催化硼氢化钠水解制氢研究[J]. 无机盐工业, 2019,51(3):72-76. |
| [3] | 孙海杰, 陈凌霞, 黄振旭, 等. 第四周期过渡金属催化硼氢化钠水解制氢研究[J]. 无机盐工业, 2017,49(5):14-17. |
| [4] | 孙海杰, 黄振旭, 王雅苹, 等. 非晶态合金Ru-B/ZrO2催化剂催化硼氢化钠水解制氢性能的研究[J]. 化工新型材料, 2018,46(1):102-105. |
| [5] | 张帅, 王斯瑶, 姜召, 等. 静电纺丝技术在氨硼烷水解脱氢催化剂制备中的应用[J]. 化工进展, 2019,38(7):3194-3206. |
| [6] | 杨晓婧, 尚伟, 李兰兰, 等. 金属催化氨硼烷制氢研究进展[J]. 电源技术, 2014,38(7):1387-1389. |
| [7] |
杨宇雯, 冯刚, 卢章辉, 等. 原位合成钴/还原氧化石墨烯纳米粒子催化氨硼烷制氢[J]. 物理化学学报, 2014,30(6):1180-1186.
doi: 10.3866/PKU.WHXB201404141 |
| [8] | 徐凤勤, 胡小飞, 程方益, 等. 多孔碳负载镍纳米颗粒的制备及催化氨硼烷水解制氢[J]. 无机化学学报, 2015,31(1):103-108. |
| [9] | 黄维, 胡娜, 桂田, 等. 沸石咪唑酯骨架结构材料Co-ZIF-9催化氨硼烷水解制氢[J]. 江西师范大学学报:自然科学版, 2015,39(4):404-410. |
| [10] | 程军, 邹勇进, 向翠丽, 等. 纳米多孔Co-Ni-B/Cu-BTC复合材料的制备及其催化水解氨硼烷的研究[J]. 功能材料, 2016,47(6):6152-6157. |
| [11] | 杨昆, 姚淇露, 卢章辉, 等. 快速合成廉价CuMo纳米粒子高效催化氨硼烷水解产氢[J]. 物理化学学报, 2017,33(5):993-1000. |
| [12] |
孙海杰, 江厚兵, 李帅辉, 等. 纳米Ru-Mn/ZrO2催化剂上苯选择加氢制环己烯[J]. 催化学报, 2013,34(4):684-694.
doi: 10.1016/S1872-2067(11)60489-0 |
| [13] | 邵阳阳, 靳惠明, 俞亮, 等. Mo掺杂Co-B非晶态合金的制备及催化硼氢化钠水解制氢性能[J]. 材料导报, 2020,34(2):2063-2066. |
| [14] |
孙海杰, 陈凌霞, 黄振旭, 等. Ru-Zn催化剂在苯选择加氢制环己烯反应中的粒径效应[J]. 高等学校化学学报, 2015,36(10):1969-1976.
doi: 10.7503/cjcu20150288 |
| [15] |
孙海杰, 陈秀丽, 黄振旭, 等. NaOH浓度对苯选择加氢制环己烯Ru-Zn催化剂性能的影响[J]. 化工学报, 2016,67(4):1324-1332.
doi: 10.11949/j.issn.0438-1157.20151370 |
| [16] |
孙海杰, 郭伟, 周小莉. 非晶态合金 Ru 基催化剂在苯选择加氢中的应用进展[J]. 催化学报, 2011,32(1):1-16.
doi: 10.1016/S1872-2067(10)60154-4 |
| [17] | 朱玉玲, 郑修成, 刘蒲. 磁性花生壳负载钯催化剂的制备及催化氨硼烷释氢性能[J]. 信阳师范学院学报:自然科学版, 2019,32(2):276-280. |
| [18] | Dong H, Yang H, Ai X, et al. Hydrogen production from catalytic hydrolysis of sodium borohydride solution using nickel boride cat-alyst[J]. Int.J.Hydrogen Energy, 2003,28(10):1095-1100. |
| [19] | 鲍新侠, 于晓飞, 李其明, 等. CoB/ZSM-5非晶态合金负载型催化剂在硼氢化钠水解制氢中的应用[J]. 应用化工, 2014,43(10):1747-1750. |
| [1] | Feng Fei,Li Shuwen,Wang Tielin,Wang Weiguo,Wang Cunwen. Synthesis and photocatalytic performance of sheet-like Bi/BiVO4 composite catalyst [J]. Inorganic Chemicals Industry, 2021, 53(1): 107-112. |
| [2] | Fu Haikuo,Wu Lijue,Wen Dingqiang,Chen Qiu,Zhong Hui,Zhang Tao,Ran Jianjun. Preparation of micron-sized cobalt hydroxide with low chlorine content [J]. Inorganic Chemicals Industry, 2021, 53(1): 40-43. |
| [3] | Cao Weiwen,Li Jun,Chen Ming,Yu Yinrong,Liao Xiaoting. Study on agglomeration process during crystallization of calcium hydrogen phosphate and its main affecting impurity thereof [J]. Inorganic Chemicals Industry, 2021, 53(1): 50-53. |
| [4] | Chen Mo,Sha Zuoliang. Study on new process for producing potassium chloride using cold-decomposition-sieving method [J]. Inorganic Chemicals Industry, 2021, 53(1): 65-67. |
| [5] | Feng Qing,Zeng Xianjun,Zhang Lijie,Li He,Meng Guangying,Sun Yanmin. Summary of transition metal manganese-based denitration catalyst for low temperature NH3-SCR [J]. Inorganic Chemicals Industry, 2021, 53(1): 7-13. |
| [6] | Hang Meiyan,Peng Yajuan,Liu Xinxin,Zhang Haiyan,Tao Xu. Quantitative study on influence of ferrochrome slag modification based on mortar strength decoupling method [J]. Inorganic Chemicals Industry, 2021, 53(1): 72-76. |
| [7] | Zhang Bin,Xiao Xiao,Han Yunjiao,Zhang Yuanyuan,Yu Hongwei. Study on third-step infrared spectroscopy of calcium carbonate [J]. Inorganic Chemicals Industry, 2021, 53(1): 97-101. |
| [8] | Gan Yonghai,Wu Bingdang,Li Haojie,Li Jingbiao,Li Runsheng,Zhang Shujuan. Preparation and application evaluation of a novel titanium coagulant [J]. Inorganic Chemicals Industry, 2020, 52(9): 1-5. |
| [9] | Yin Wei,Huang Jin,Xie Tian,He Bingbing. Experimental study on high efficiency decomposition of phosphate rock by phosphoric acid [J]. Inorganic Chemicals Industry, 2020, 52(9): 34-36. |
| [10] | Liu Yonghe,Zhang Yanyan. Effect of modification process of thermal conductive alumina on thermal conductivity of interfacial materials [J]. Inorganic Chemicals Industry, 2020, 52(9): 70-72. |
| [11] | Yang Xi,Zhu Jian,Liu Fang,Yang Jinxiu. Effect of modified electrolytic manganese slag substrate on growth of pasture and leaching migration of Mn and Cd [J]. Inorganic Chemicals Industry, 2020, 52(9): 73-78. |
| [12] | Lei Shaocheng,Li Yulin. Experimental study on salt separation of high salt wastewater from coal chemical HERO by DTNF nanofiltration membrane [J]. Inorganic Chemicals Industry, 2020, 52(9): 84-87. |
| [13] | Teng Xiaohui,Sun Liyue,Zhuang Lijun. Study on preparation of metallurgical grade chromium hydroxide by removing silicon from vanadium wastewater [J]. Inorganic Chemicals Industry, 2020, 52(9): 88-90. |
| [14] | Zhao Shihao,Lin Xihua,Ma Pengfei,Yuan Yijin,Gao Yuxin,Zhang Ping,Zhou Dali. Study on formulation calculation of phosphogypsum-based composite cementitious materials based on expansion and failure of critical AFt [J]. Inorganic Chemicals Industry, 2020, 52(9): 91-95. |
| [15] | Chen Lijia,Chen Haibin,Li Rongyong. Application progress of mechanical coating technique in preparation of photocatalytic materials [J]. Inorganic Chemicals Industry, 2020, 52(8): 1-5. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|