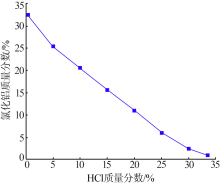
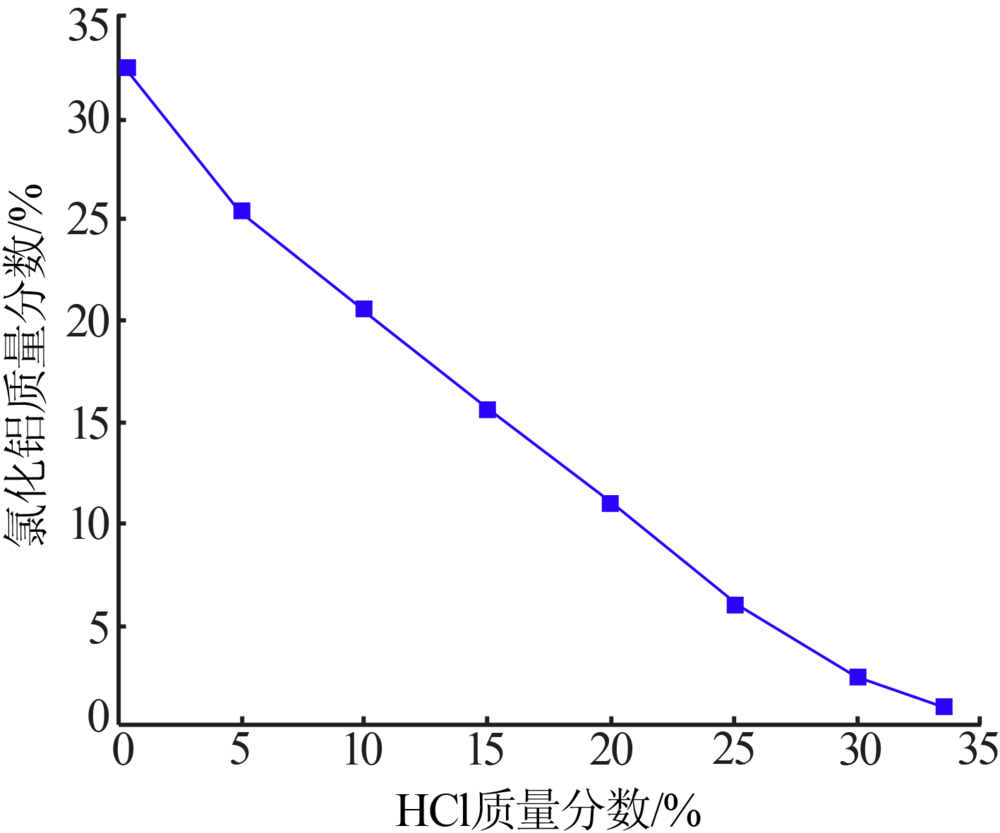
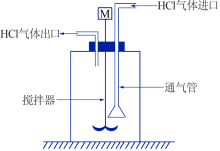
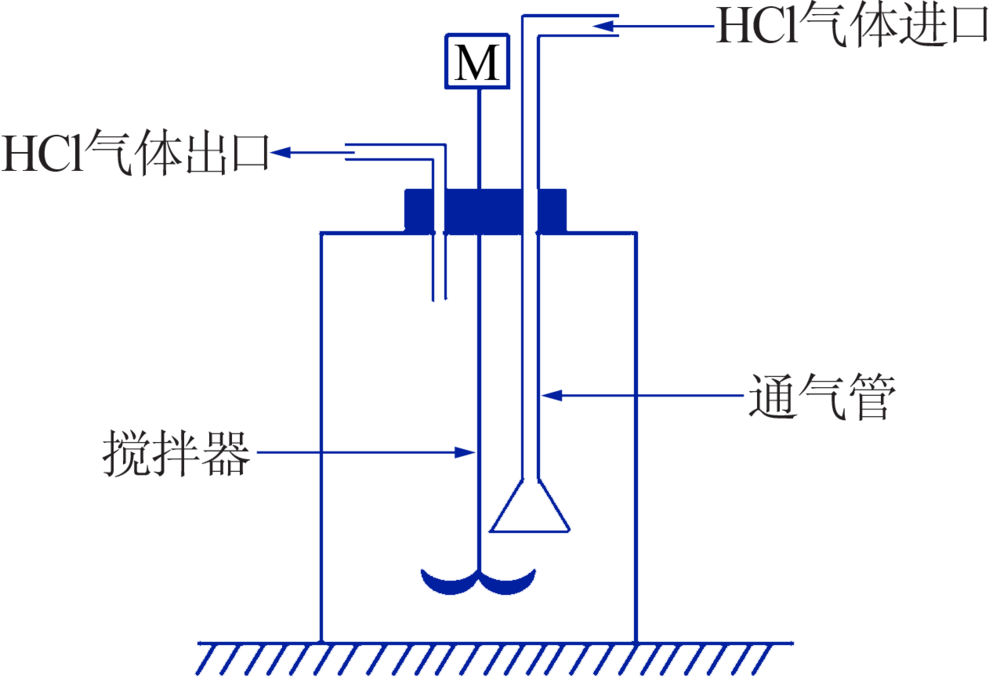
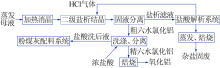
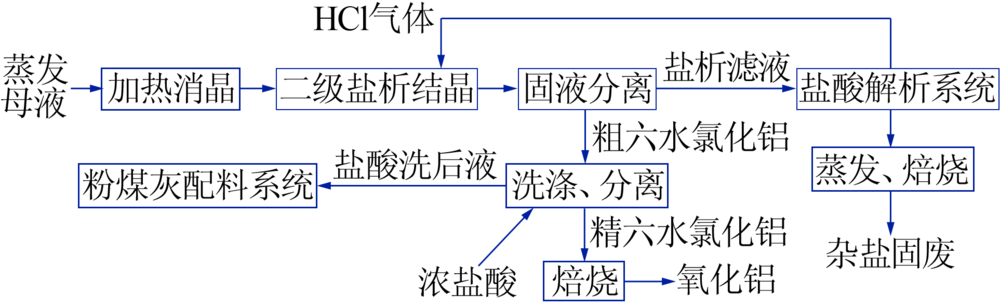
Inorganic Chemicals Industry ›› 2020, Vol. 52 ›› Issue (12): 64-68.doi: 10.11962/1006-4990.2020-0278
• Environment·Health·Safety • Previous Articles Next Articles
Study on technology of recovering aluminum chloride from evaporation mother liquor in extraction of alumina from fly ash by hydrochloric acid method
Chao Xiaoguang( ),Wang Hongbin,Cao Kun,Dai Yin,Song Litao,Zou Ping,Jia Min,Gao Guimei
),Wang Hongbin,Cao Kun,Dai Yin,Song Litao,Zou Ping,Jia Min,Gao Guimei
- Shenhua Zhunneng Resources Comprehensive Development Company Limited,Erdos 010300,China
-
Received:2020-06-20Online:2020-12-10Published:2020-12-15
CLC Number:
Cite this article
Chao Xiaoguang,Wang Hongbin,Cao Kun,Dai Yin,Song Litao,Zou Ping,Jia Min,Gao Guimei. Study on technology of recovering aluminum chloride from evaporation mother liquor in extraction of alumina from fly ash by hydrochloric acid method[J]. Inorganic Chemicals Industry, 2020, 52(12): 64-68.
share this article
"
| 样品 编号 | Al | Ca | Fe | Mg | P | Ti | Li | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 质量/ g | 析出率/ % | 质量/ g | 析出率/ % | 质量/ g | 析出率/ % | 质量/ g | 析出率/ % | 质量/ g | 析出率/ % | 质量/ g | 析出率/ % | 质量/ g | 析出率/ % | |
| 盐析原液 | 52 389.6 | — | 3 805.3 | — | 33.8 | — | 2 810.4 | — | 1 372.8 | — | 19.90 | — | 135.6 | — |
| 盐析产品-1 | 41 285.9 | 78.81 | 15.1 | 0.40 | 0.7 | 1.99 | 169.2 | 6.02 | 57.9 | 4.22 | 0.05 | 0.24 | 2.6 | 1.93 |
| 盐析产品-2 | 43 003.7 | 82.08 | 145.2 | 3.82 | 0.5 | 1.55 | 179.8 | 6.40 | 103.4 | 7.53 | 1.00 | 5.01 | 27.3 | 20.16 |
| 盐析产品-3 | 49 643.9 | 94.76 | 160.4 | 4.22 | 1.5 | 4.33 | 281.9 | 10.03 | 118.5 | 8.63 | 0.80 | 3.96 | 24.0 | 17.70 |
| 样品 编号 | Si | Zn | Cr | Cu | V | Mn | ||||||||
| 质量/ g | 析出率/ % | 质量/ g | 析出率/ % | 质量/ g | 析出率/ % | 质量/ g | 析出率/ % | 质量/ g | 析出率/ % | 质量/ g | 析出率/ % | |||
| 盐析原液 | 150.0 | — | 23.2 | — | 63.1 | — | 16.0 | — | 170.4 | — | 293.8 | — | ||
| 盐析产品-1 | 12.3 | 8.22 | 0.2 | 0.74 | 3.9 | 6.14 | 0.2 | 0.97 | 0.2 | 0.09 | 2.8 | 0.95 | ||
| 盐析产品-2 | 12.6 | 8.39 | 0.4 | 1.62 | 6.7 | 10.69 | 0.5 | 3.06 | 3.1 | 1.82 | 10.2 | 3.48 | ||
| 盐析产品-3 | 12.5 | 8.32 | 0.4 | 1.61 | 9.1 | 14.48 | 0.5 | 3.39 | 1.6 | 0.94 | 13.7 | 4.65 | ||
"
| 样品名称 | CaO | Fe2O3 | MgO | P2O5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | |
| 盐析产品-1 | 1 248 | 270 | 78 | 140 | 12 | 91 | 9 047 | 3 605 | 60 | 3 557 | 1 696 | 52 |
| 盐析产品-2 | 13 666 | 2 496 | 82 | 117 | 9 | 92 | 13 669 | 3 677 | 73 | 9 281 | 2 905 | 69 |
| 盐析产品-3 | 9 521 | 2 388 | 75 | 85 | 22 | 74 | 15 213 | 4 996 | 67 | 7 106 | 2 885 | 59 |
| 样品名称 | TiO2 | Li2O | SiO2 | ZnO | ||||||||
| 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | |
| 盐析产品-1 | 8 | 1 | 87 | 978 | 72 | 93 | 441 | 338 | 23 | 13 | 3 | 79 |
| 盐析产品-2 | 101 | 20 | 80 | 4 093 | 719 | 82 | 562 | 331 | 41 | 53 | 6 | 89 |
| 盐析产品-3 | 67 | 14 | 79 | 1 677 | 547 | 67 | 624 | 284 | 54 | 32 | 5 | 85 |
| 样品名称 | Cr2O3 | CuO | V2O5 | MnO2 | ||||||||
| 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | |
| 盐析产品-1 | 131 | 72 | 45 | 23 | 2 | 89 | 27 | 2 | 91 | 397 | 56 | 86 |
| 盐析产品-2 | 283 | 121 | 57 | 46 | 8 | 84 | 519 | 45 | 91 | 1 241 | 198 | 84 |
| 盐析产品-3 | 249 | 142 | 43 | 11 | 7 | 32 | 372 | 20 | 95 | 869 | 230 | 74 |
"
| 样品名称 | 质量分数/% | 烧失量/% | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | CaO | Fe2O3 | MgO | P2O5 | TiO2 | Li2O | SiO2 | ZnO | Cr2O3 | CuO | V2O5 | MnO2 | |||
| 盐析 产品-1 | 洗前 | 95.07 | 1.367 | 0.012 | 1.367 | 0.928 | 0.010 | 0.409 | 0.056 | 0.005 | 0.028 | 0.005 | 0.052 | 0.124 | 0.562 |
| 洗后 | 98.40 | 0.250 | 0.001 | 0.368 | 0.291 | 0.002 | 0.072 | 0.033 | 0.001 | 0.012 | 0.001 | 0.004 | 0.020 | 0.543 | |
| 盐析 产品-2 | 洗前 | 95.87 | 0.952 | 0.008 | 1.521 | 0.711 | 0.007 | 0.168 | 0.062 | 0.003 | 0.025 | 0.001 | 0.037 | 0.087 | 0.551 |
| 洗后 | 98.29 | 0.239 | 0.002 | 0.500 | 0.289 | 0.001 | 0.055 | 0.028 | 0.000 | 0.014 | 0.001 | 0.002 | 0.023 | 0.553 | |
| 盐析 产品-3 | 洗前 | 97.87 | 0.125 | 0.014 | 0.905 | 0.356 | 0.001 | 0.098 | 0.044 | 0.001 | 0.013 | 0.002 | 0.003 | 0.040 | 0.526 |
| 洗后 | 98.86 | 0.027 | 0.001 | 0.361 | 0.170 | 0.000 | 0.007 | 0.034 | 0.000 | 0.007 | 0.000 | 0.000 | 0.006 | 0.531 | |
"
| 样品名称 | CaO | Fe2O3 | MgO | P2O5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | |
| 二级盐析产品 | 231.06 | 26.80 | 88.4 | 39.82 | 7.98 | 80.0 | 700.78 | 284.87 | 59.3 | 455.51 | 205.10 | 55.0 |
| 样品名称 | TiO2 | Li2O | SiO2 | ZnO | ||||||||
| 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | |
| 二级盐析产品 | 1.66 | 0.33 | 80.0 | 87.45 | 35.47 | 59.4 | 295.64 | 179.50 | 39.3 | 1.49 | 0.50 | 66.4 |
| 样品名称 | Cr2O3 | CuO | V2O5 | MnO2 | ||||||||
| 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | 洗前 w/10-6 | 洗后 w/10-6 | 洗涤 效率/% | |
| 二级盐析产品 | 28.51 | 11.37 | 60.1 | 1.24 | 0.25 | 80.0 | 5.33 | 0.36 | 93.3 | 11.97 | 0.00 | 100.0 |
| [1] | Felker K, Seeley F, Egan Z, et al. Aluminum from fly ash[J]. Chemmtech, 1982,12(2):123-128. |
| [2] | Blissett R S, Rowson N A. A review of the multi-component utilisation of coal fly ash[J]. Fuel, 2012,97:1-23. |
| [3] | Park H C, Park Y J. Synjournal of alumina from high purity alum devived from coal fly ash[J]. Mater.Sci.Eng.A, 2004(367):166-170. |
| [4] | Zhang B Y, Zhou F L. The limestone sintering process to produce alumina with fly ash[J]. Light Metals, 2007(6):17-18. |
| [5] | 蒋训雄, 蒋开喜, 汪胜东, 等. 酸碱联合综合回收高铝粉煤灰中氧化铝和氧化硅[J]. 有色金属:冶炼部分, 2019(9):28-32. |
| [6] | Brown R R. Solubility and activity of aluminium chloride in hydrochloric acid solutions[R]. US Bur.Mines Rep.RI 8379, 1979. |
| [7] | Gokcen N A. Partial Pressures of Gaseous HCl and H2O over aqueous solutions of HCl,A1Cl3,and FeCl3[R]. US Bur.Mines Rep. RI8456, 1980. |
| [8] | Eisele J A. Producing alumina from clay by the hydrochloric acid process,a bench study[R]. US Bur.Mines,Rep.RI 8476, 1980. |
| [9] | Maysilles J H, Traut D E. Aluminium chloride hexahydrate crystallisation by HC1 gas sparging[R]. US Bur.Mines Rep.RI 8590, 1981. |
| [10] | Shanks D E, Eisele J A, Bauer D J. Hydrogen chloride sparging crystallisation of aluminium chloride hexahydrate crystallisation[R]. US Bur.Mines Rep.RI 8593, 1981. |
| [11] | Sorensen R T, Sawyer D L. Alumina miniplant operations separation of aluminum chloride liquor from leach residue solids by classification and thickening[R]. US Bur.Mines Rep.RI 8805, 1983. |
| [12] | Livingston W R. The use of coal spoils as feed materials for alumina recovery by acid-leaching routes.1.The suitability and variability of the feed materials[J]. Hydrometallurgy, 1983,10:79-96. |
| [13] | Livingston W R, Rogers D A, Chapman R J, et al. The use of coal spoils as feed materials for alumina recovery by acid-leaching routes:2.The effect of the calcination conditions on the leaching properties of the colliery spoil[J]. Hydrometallurgy, 1983,10:97-109. |
| [14] | Livingston W R, Rogers D A. The use of coal spoils as feed materials for alumina recovery by acid-leaching routes.3.The effect of the leaching conditions on the extraction of aluminium and iron from a fluidised bed ash[J]. Hydrometallurgy, 1985,13:283-291. |
| [15] | Mahi P, Bailey N T. The use of coal spoils as feed materials for alumina recovery by acid-leaching routes.4.The extraction of iron from aluminiferous solutions with amines,in particular Alamine 336[J]. Hydrometallurgy, 1985,13:293-304. |
| [16] | Bailey N T, Chapman R J. The use of coal spoils as feed materials for alumina recovery by acid-leaching routes.5.The effect of fluoride addition on the extraction of aluminium with hydrochloric acid[J]. Hydrometallurgy, 1987,18:337-350. |
| [17] | Mahi P, Livingston W R. The use of coal spoils as feed materials for alumina recovery by acid-leaching routes.6.The purification and crystallisation of chloride and chloride/fluoride leach liquors by HCl gas precipitation[J]. Hydrometallurgy, 1991,26:75-91. |
| [18] | Marchessaux P. Thermal decomposition of aluminium hexahydrate chloride for alumina production[J]. Light Metal, 1979(4):189-205. |
| [19] | Orbite Aluminae INC. Processes for extracting aluminum from aluminous ores:US,8337789[P]. 2012-12-25. |
| [20] | Orbite Aluminae INC. Processes for recovering rare earth elements from various materials:CA,2013170370[P]. 2013-05-16. |
| [21] | 郭昭华. 粉煤灰“一步酸溶法”提取氧化铝工艺技术及工业化发展研究[J]. 煤炭工程, 2015,47(7):5-8. |
| [22] | 钞晓光. 粉煤灰“一步酸溶法”提取氧化铝工业化除杂技术的研究开发[J]. 中国化工贸易, 2017,1(9):109-113. |
| [23] | 郭昭华, 王宏宾, 王永旺, 等. 一种用于氯化铝溶液除钙的方法:中国,107628633A[P]. 2018-01-26. |
| [1] | LIU Xinlong, YANG Zhenyu, HAO He, LIU Shuxin, WU Chenyang, WANG Xingli, MA Qingqing. Study on shaped 4A zeolite synthesized with aluminum extraction residue by fly ash [J]. Inorganic Chemicals Industry, 2025, 57(3): 78-85. |
| [2] | LI Chao, WANG Liping, GAO Guimei, ZHANG Yunfeng, HONG Yu, LIU Darui, XU Lijun, CUI Yongjie. Study on reaction mechanism of acid leaching lithium from circulating fluidized bed fly ash [J]. Inorganic Chemicals Industry, 2025, 57(3): 101-107. |
| [3] | ZHAO Feiyan, ZHANG Xiaodong, DU Yanxia, WANG Qiang, LI Xiaoyan. Preparation technology and research progress of fly ash ceramsite [J]. Inorganic Chemicals Industry, 2024, 56(4): 16-23. |
| [4] | LI Kuai, LI Zhaoshuai, DONG Tingxuan, LI Dan, GUO Shengwei, HAN Fenglan. Study on effect of wet magnetic separation on distribution of Fe and heavy metal in fly ash [J]. Inorganic Chemicals Industry, 2024, 56(4): 98-104. |
| [5] | LI Qiaoyun, HUANG Xiuxing, WEI Wenye, CHEN Zhen. Study on adsorption of methylene blue by activated carbon with acid/alkali synergistically modified fly ash [J]. Inorganic Chemicals Industry, 2024, 56(3): 131-136. |
| [6] | ZHAO Shiyong, XIAO Yuchen, MA Qingqing, YANG Zhenni, WANG Jizhen, FAN Xiaoping. Study on adsorption of Cu(Ⅱ) on 4A zeolite synthesized by aluminum extraction residue by fly ash [J]. Inorganic Chemicals Industry, 2024, 56(10): 127-134. |
| [7] | LI Wen, WANG Wenxiang, FANG Hongsheng, WU Pingxiao. Study on effect mechanism of silicon-aluminum additives on stabilization of heavy metals in fly ash by mechanochemical stabilization method [J]. Inorganic Chemicals Industry, 2023, 55(4): 84-91. |
| [8] | SUN Zhigao, WU Yuan, YANG Xingchun, ZHANG Dongliang, WANG Mitang. Preparation and properties of high-magnesium nickel slag-fly ash based geopolymer [J]. Inorganic Chemicals Industry, 2023, 55(11): 139-146. |
| [9] | YANG Hongjun, WANG Min, GE Haiwen, QIAO Youmin, QIAO Ziyang. Study on recycling process of potassium from calcium aluminate fly ash [J]. Inorganic Chemicals Industry, 2023, 55(10): 121-127. |
| [10] | HE Wenchao,XUE Jing,WANG Wei. Research on strength and creep characteristics of concrete containing fly ash microbead [J]. Inorganic Chemicals Industry, 2023, 55(1): 124-128. |
| [11] | LIU Darui,XU Lijun,LI Shichun,CAO Kun,TU Ya,LI Wenqing,LIU Qingliang. Research progress of recovery of strategic metal lithium from fly ash [J]. Inorganic Chemicals Industry, 2023, 55(1): 56-63. |
| [12] | LEI Ming,ZHU Hanyu,LIU Zilong,CHEN Guopeng,YUAN Junsheng. Distribution and speciation of heavy metals in hardened solid of net slurry co?disposed by cement kiln [J]. Inorganic Chemicals Industry, 2022, 54(8): 107-113. |
| [13] | CUI Jiaxin,WANG Lianyong,LU Simeng,SUN Yanwen,WANG Rui,HE Yan,HAN Jianli. Research on performance of hydrothermally synthesized zeolite with fly ash from different producing areas [J]. Inorganic Chemicals Industry, 2022, 54(5): 96-100. |
| [14] | CUI Jiaxin,WANG Lianyong,LI Yao,HE Yan,WANG Rui,HAN Jianli. Preparation and properties characterization of water quenching slag-fly ash based 4A zeolite [J]. Inorganic Chemicals Industry, 2022, 54(4): 135-140. |
| [15] | LI Yajiao,ZHAO Yiwei,JU Kai,TANG Renlong,LI Longqing,SHAO Xiaoping,ZHANG Gaofeng,REN Wuang. Study on optimization of extraction conditions in process of determination of ammonia content in fly ash based on response surface method [J]. Inorganic Chemicals Industry, 2022, 54(4): 145-151. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||