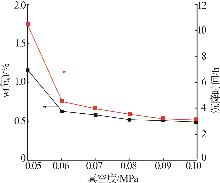
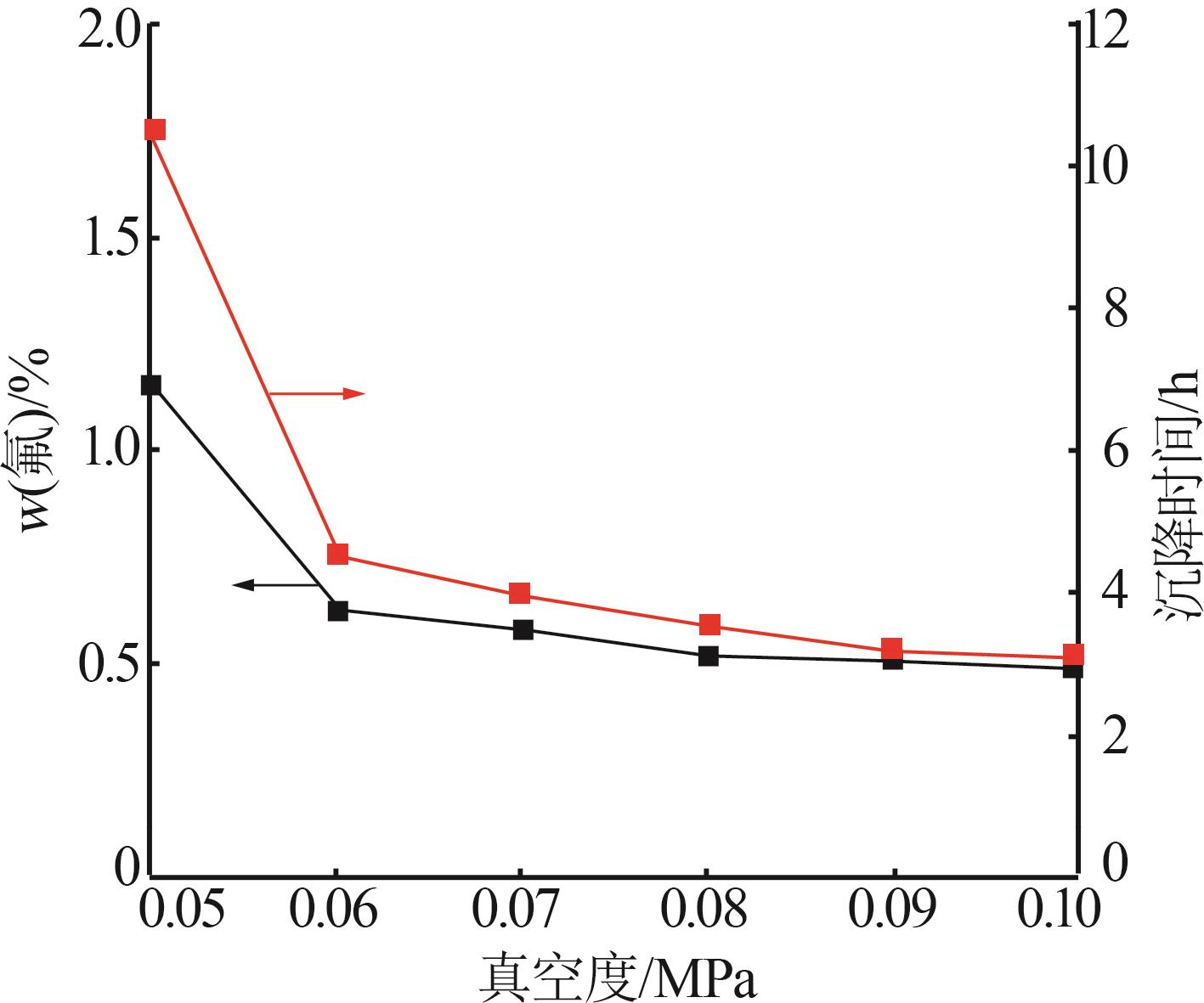
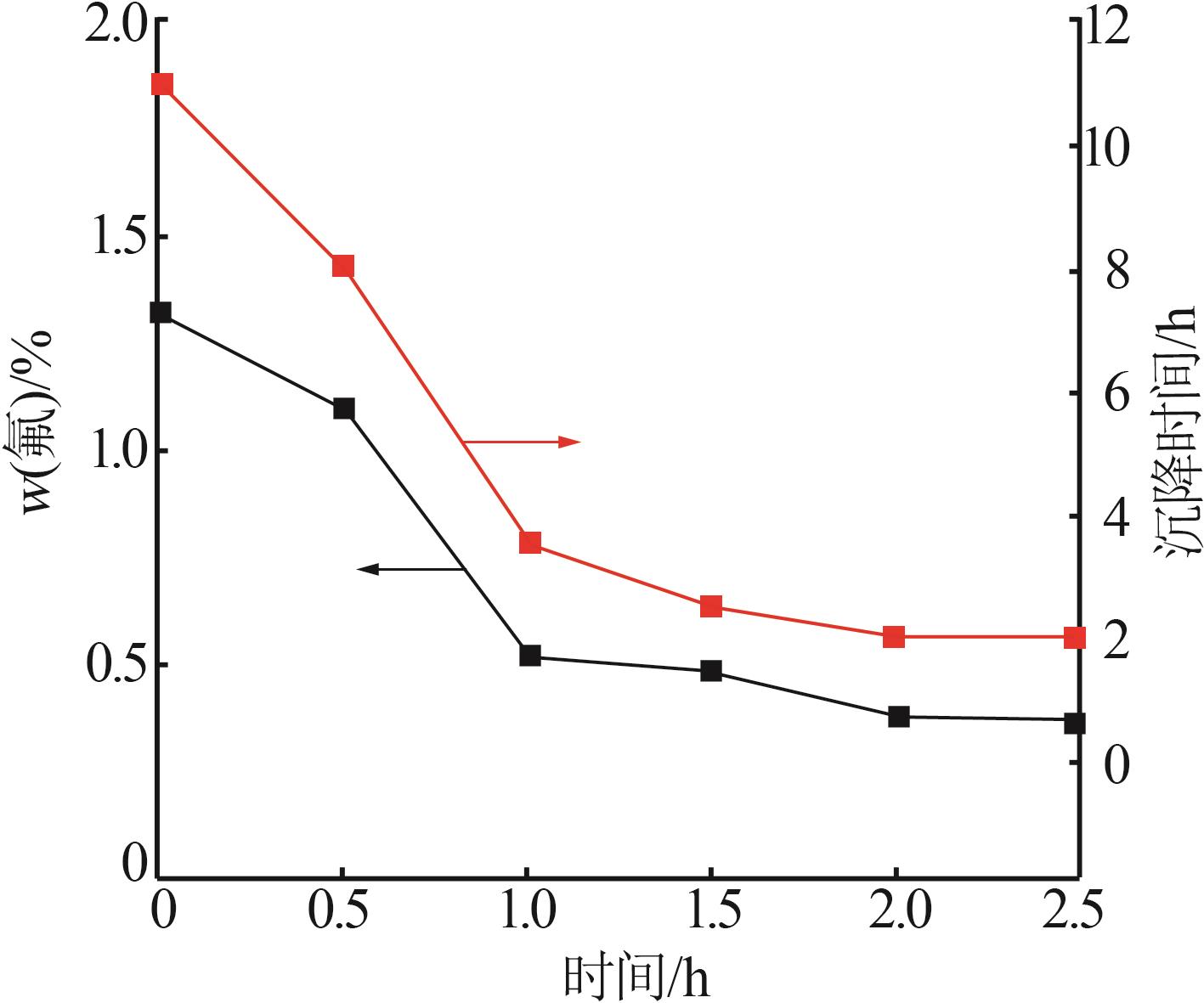
| [1] |
何宾宾.对我国磷氟协同发展的思考与建议[J].云南化工,2024,51(S1):20-24.
|
|
HE Binbin.Thoughts and suggestions on the coordinated development of phosphorus and fluorine in China[J].Yunnan Chemical Technology,2024,51(S1):20-24.
|
| [2] |
薛河南.湿法磷酸预处理工艺实验研究及技术应用[J].磷肥与复肥,2020,35(5):27-30.
|
|
XUE Henan.Experimental study and technical application of WPA pretreatment process[J].Phosphate & Compound Fertilizer,2020,35(5):27-30.
|
| [3] |
王佩.湿法磷酸氨化料浆的絮凝沉降及其分离的研究[D].武汉:武汉工程大学,2017.
|
|
WANG Pei.Study on the flocculation,sedimentation and separation of the ammoniated slurry of wet phosphoric acid[D].Wuhan:Wuhan Institute of Technology,2017.
|
| [4] |
李建闻,解秘,朱桂华.不同离子型聚丙烯酰胺在湿法磷酸生产中的应用研究[J].磷肥与复肥,2023,38(11):31-33.
|
|
LI Jianwen, XIE Mi, ZHU Guihua.Application of different ionic polyacrylamide in wet-process phosphoric acid production[J].Phosphate & Compound Fertilizer,2023,38(11):31-33.
|
| [5] |
CHEN Shuichang, LI Shiqian, WU Wanghua,et al.To treat wastewater with wastes:A highly efficient flocculant from fly ash and rice straw[J].RSC Advances,2024,14(44):32389-32397.
|
| [6] |
QIU Jinghong, WANG Hui, DU Zongliang,et al.Preparation of polyacrylamide via dispersion polymerization with gelatin as a stabilizer and its synergistic effect on organic dye flocculation[J].Journal of Applied Polymer Science,2018,135(21):46298.
|
| [7] |
HE Binbin, ZHU Yuanzhi, ZU Yun,et al.Designing an efficient fluorine recovery strategy for wet-process phosphoric acid purification by disclosing competitive complexation behavior between fluorine species and metal cations[J].Separation and Purification Technology,2023,320:124219.
|
| [8] |
HE Binbin, ZHU Yuanzhi, ZU Yun,et al.Efficient and cost-effective fluorine recovery from liquid-phase wet-process phosphoric acid via two-step precipitation method[J].Separation and Purification Technology,2023,325:124687.
|
| [9] |
ANOUAR I, JOURAIPHY R, MAZOUZ H,et al.Enhancing wet phosphoric acid production efficiency with vanadium catalyst waste derived silica[J].Heliyon,2024,10(21):e38968.
|
| [10] |
何宾宾,周琼波,张晖,等.湿法磷酸汽提法脱氟技术研究[J].无机盐工业,2016,48(9):49-50.
|
|
HE Binbin, ZHOU Qiongbo, ZHANG Hui,et al.Study on defluorination for purifying wet-process phosphoric acid by steam stripping[J].Inorganic Chemicals Industry,2016,48(9):49-50.
|
| [11] |
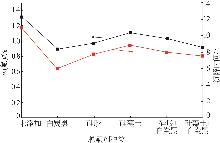
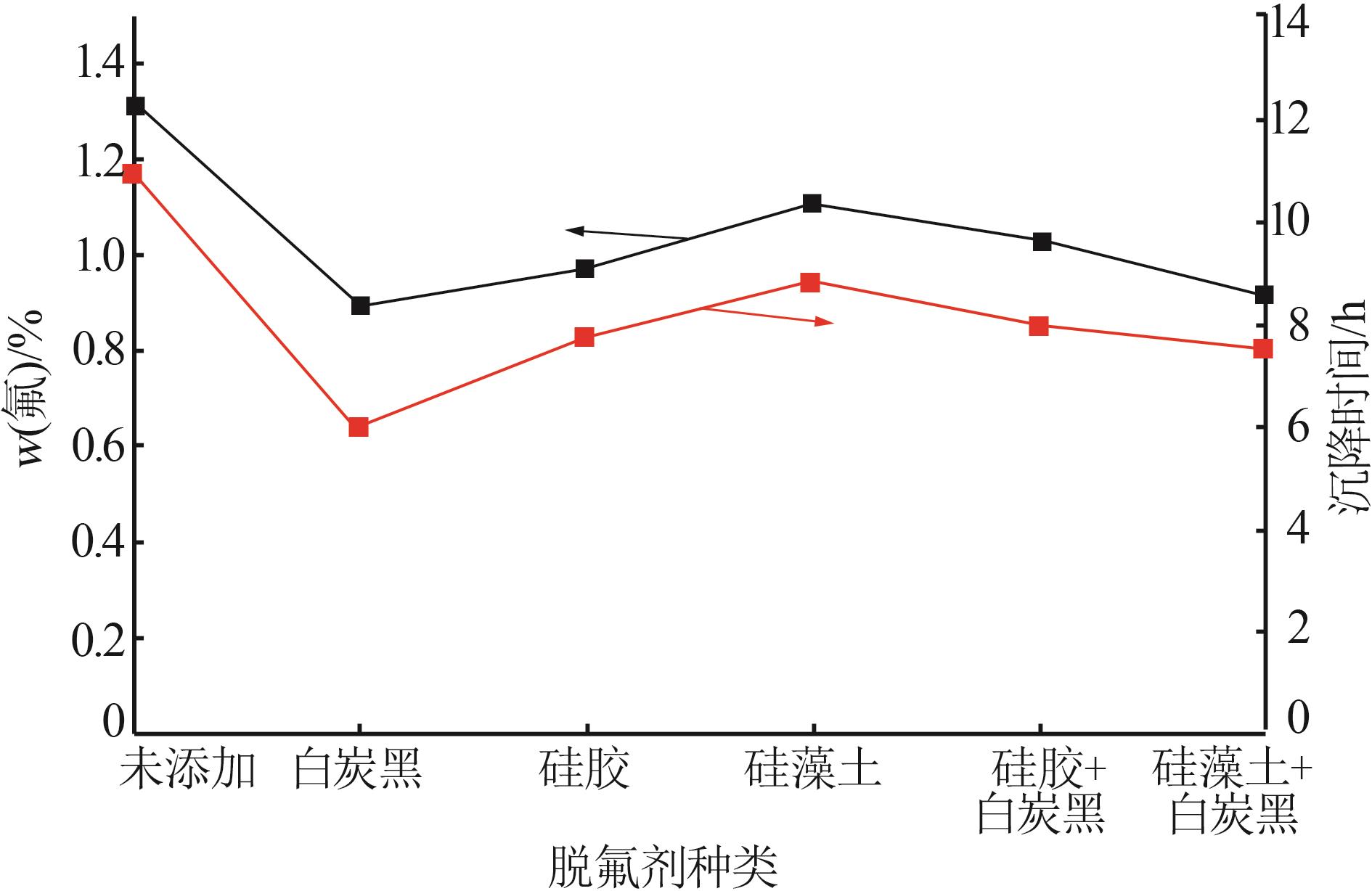
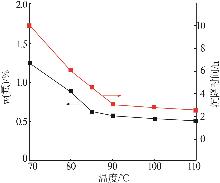
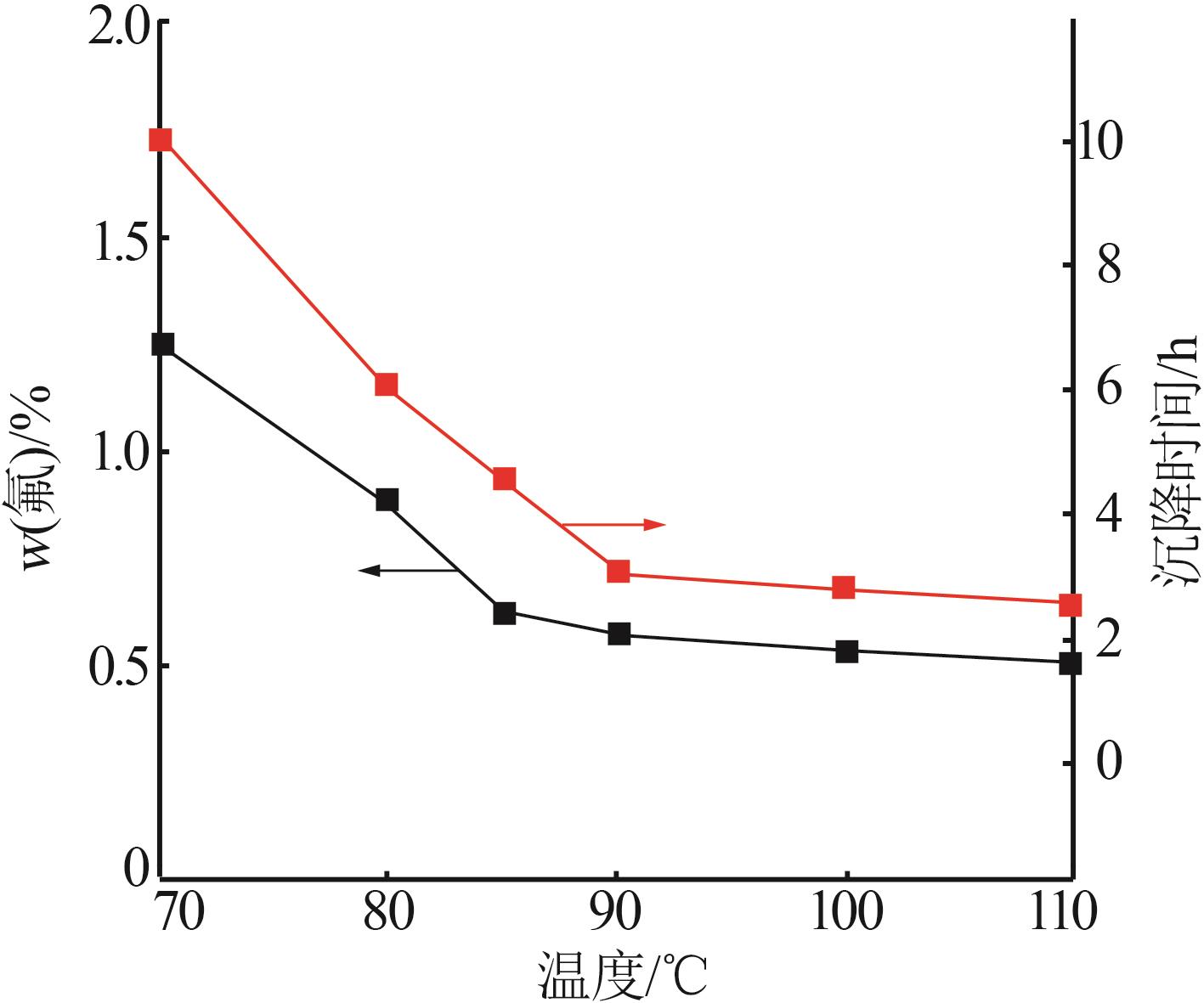
张正,刘明高,李防,等.湿法磷酸生产中沉降剂的研究及应用[J].山东化工,2020,49(14):38-39,43.
|
|
ZHANG Zheng, LIU Minggao, LI Fang,et al.Application of flocculant in production of WPA[J].Shandong Chemical Industry,2020,49(14):38-39,43.
|
| [12] |
张利锋,王凯,王晓飞,等.硅藻土衍生硅碳负极材料的制备及其储锂性能研究[J].陕西科技大学学报,2024,42(5):119-125.
|
|
ZHANG Lifeng, WANG Kai, WANG Xiaofei,et al.Study on preparation of diatomite-derived silicon carbon anode material and its lithium storage performance[J].Journal of Shaanxi University of Science & Technology,2024,42(5):119-125.
|
| [13] |
李霁,欧卓华,曹仲,等. Fe3O4/MoS2改性生物炭活化过硫酸盐协同单宁酸调理污泥[J].环境科学与技术,2024,47(8):35-43.
|
|
LI Ji, Zhuohua OU, CAO Zhong,et al.Tannic acid coupled with Fe3O4/MoS2 modified biochar to activate permonosulfate for improving sludge dewaterability[J].Environmental Science & Technology,2024,47(8):35-43.
|
| [14] |
HE Binbin, ZHU Yuanzhi, XIE Delong,et al.Removal of Al3+ and Mg2+ ions in wet-process phosphoric acid via the formation of aluminofluoride complexes[J].Environmental Technology,2023,44(7):936-947.
|
 ), GONG Li1,2, FU Yizhong1, JIANG Wei1,2, TU Zhongbing1,2, XIE Mi1, ZHU Guihua1,2(
), GONG Li1,2, FU Yizhong1, JIANG Wei1,2, TU Zhongbing1,2, XIE Mi1, ZHU Guihua1,2( )
)