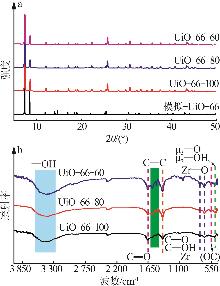
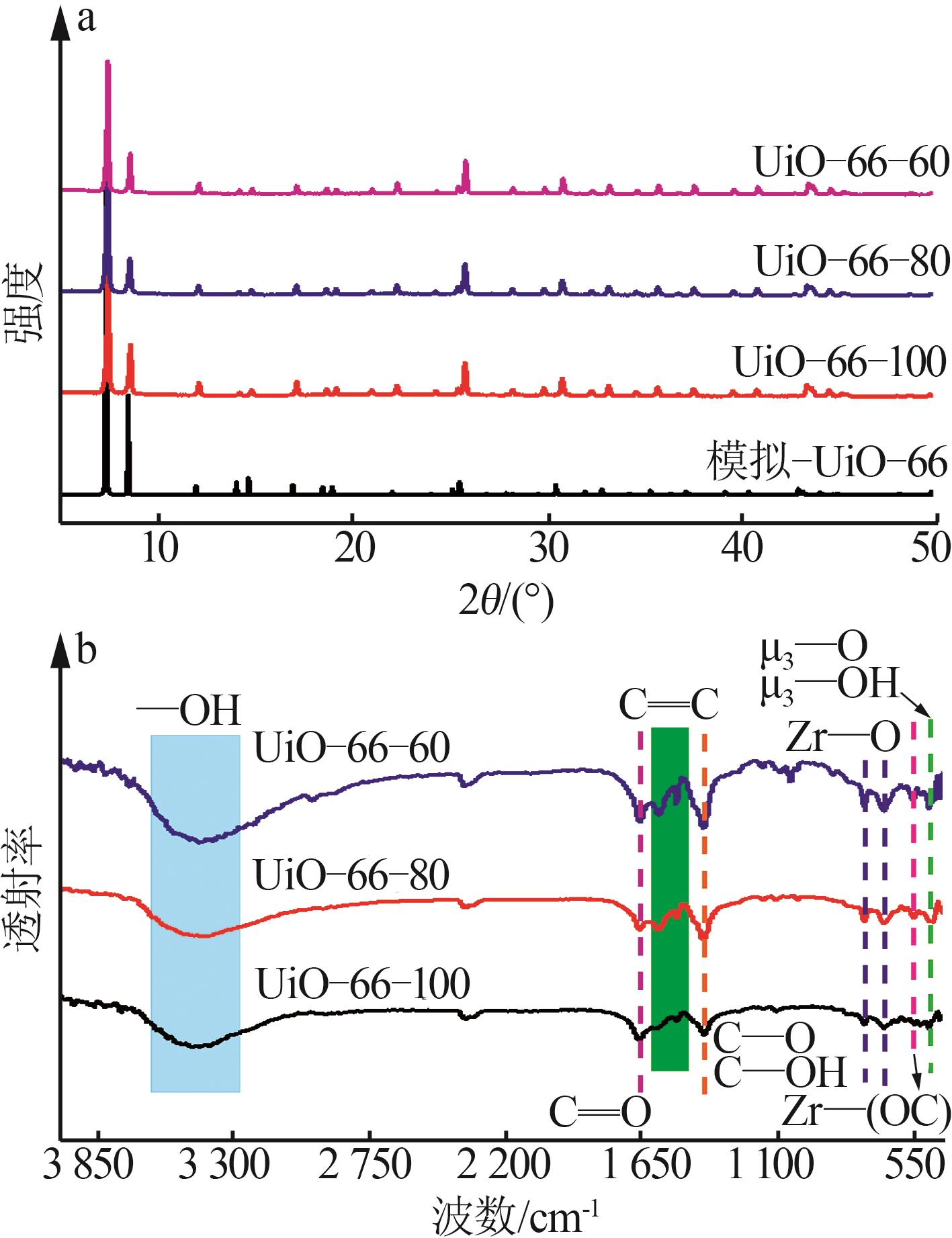
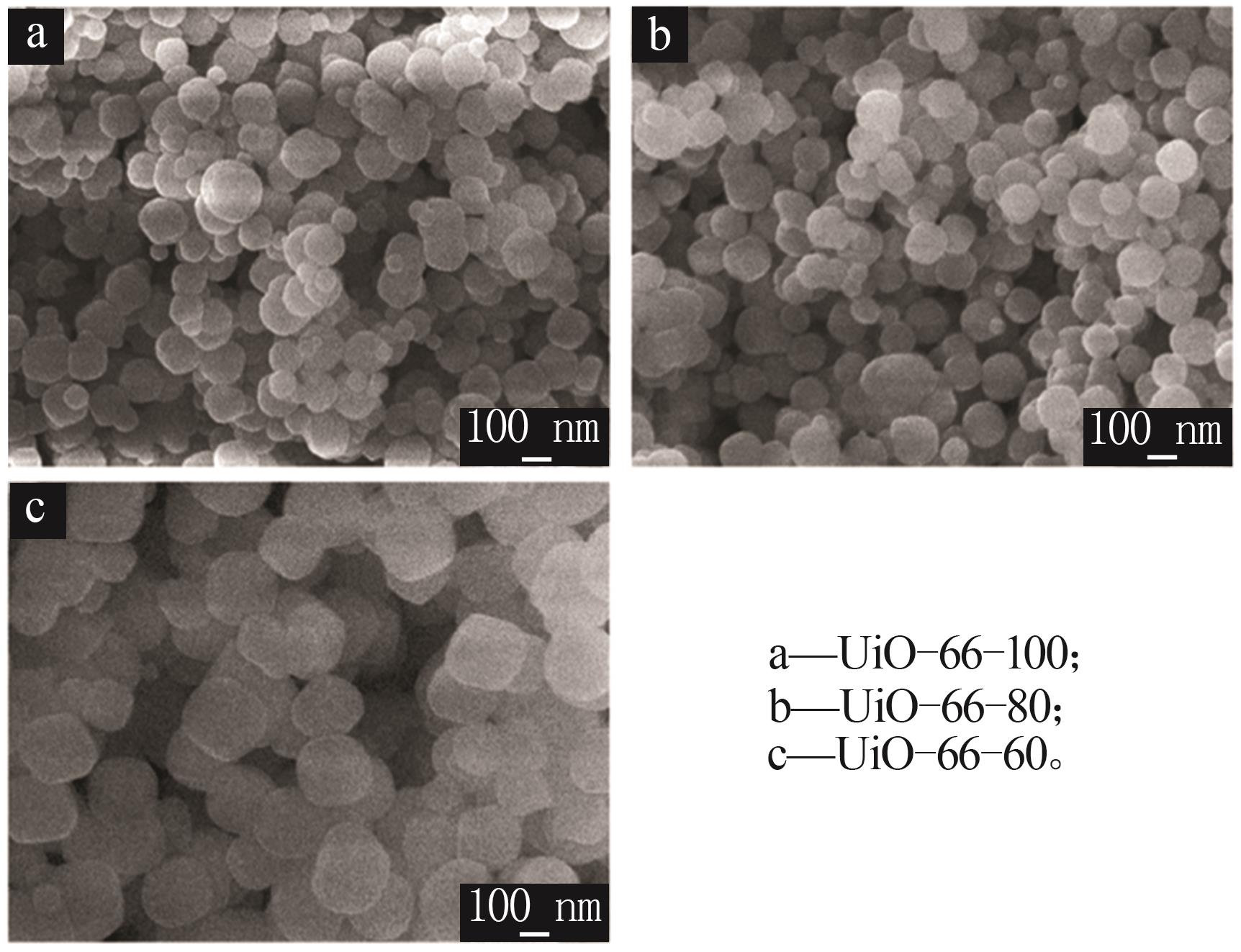
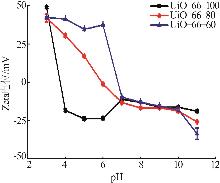
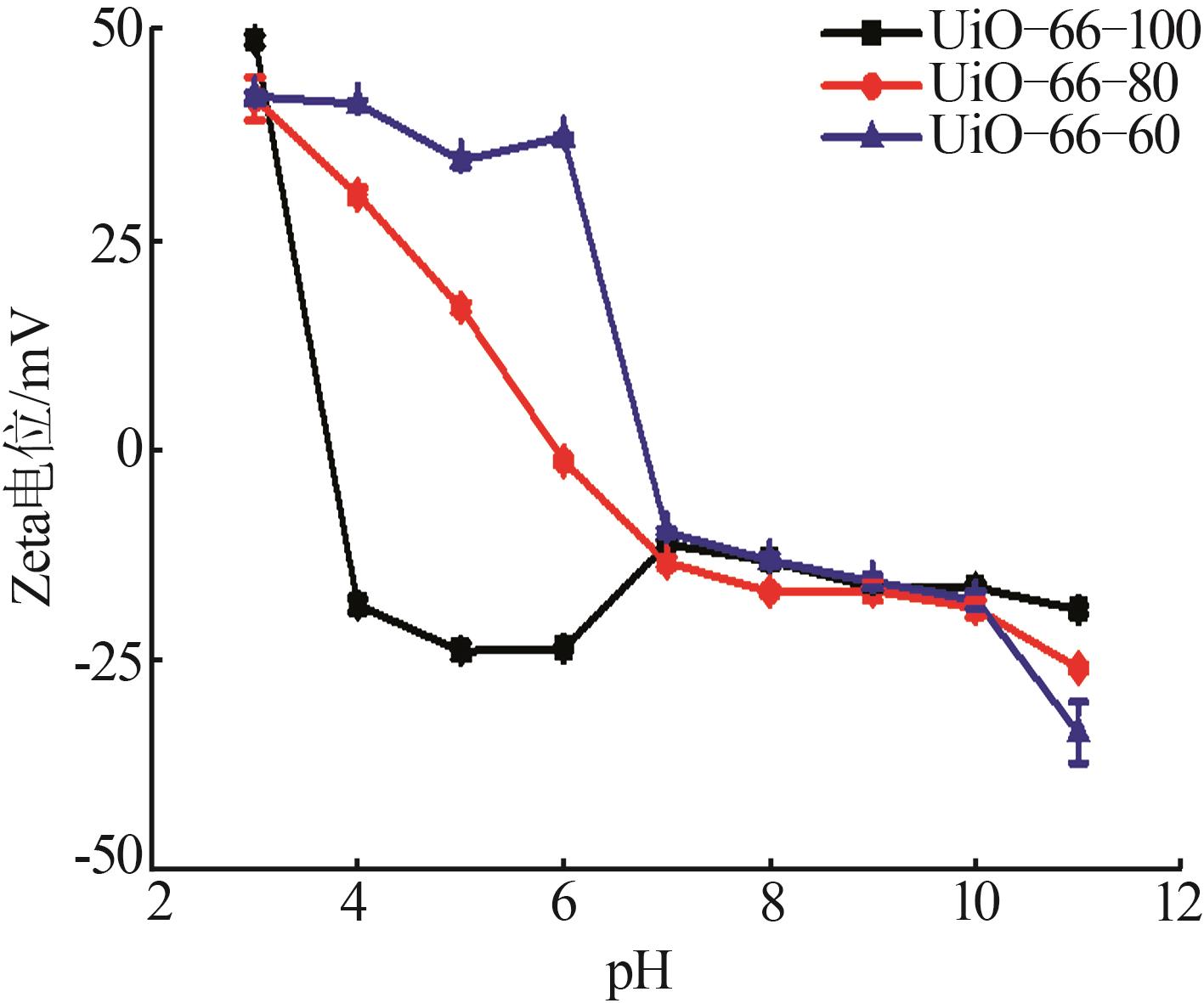
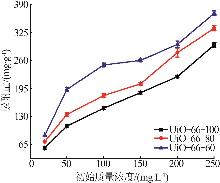
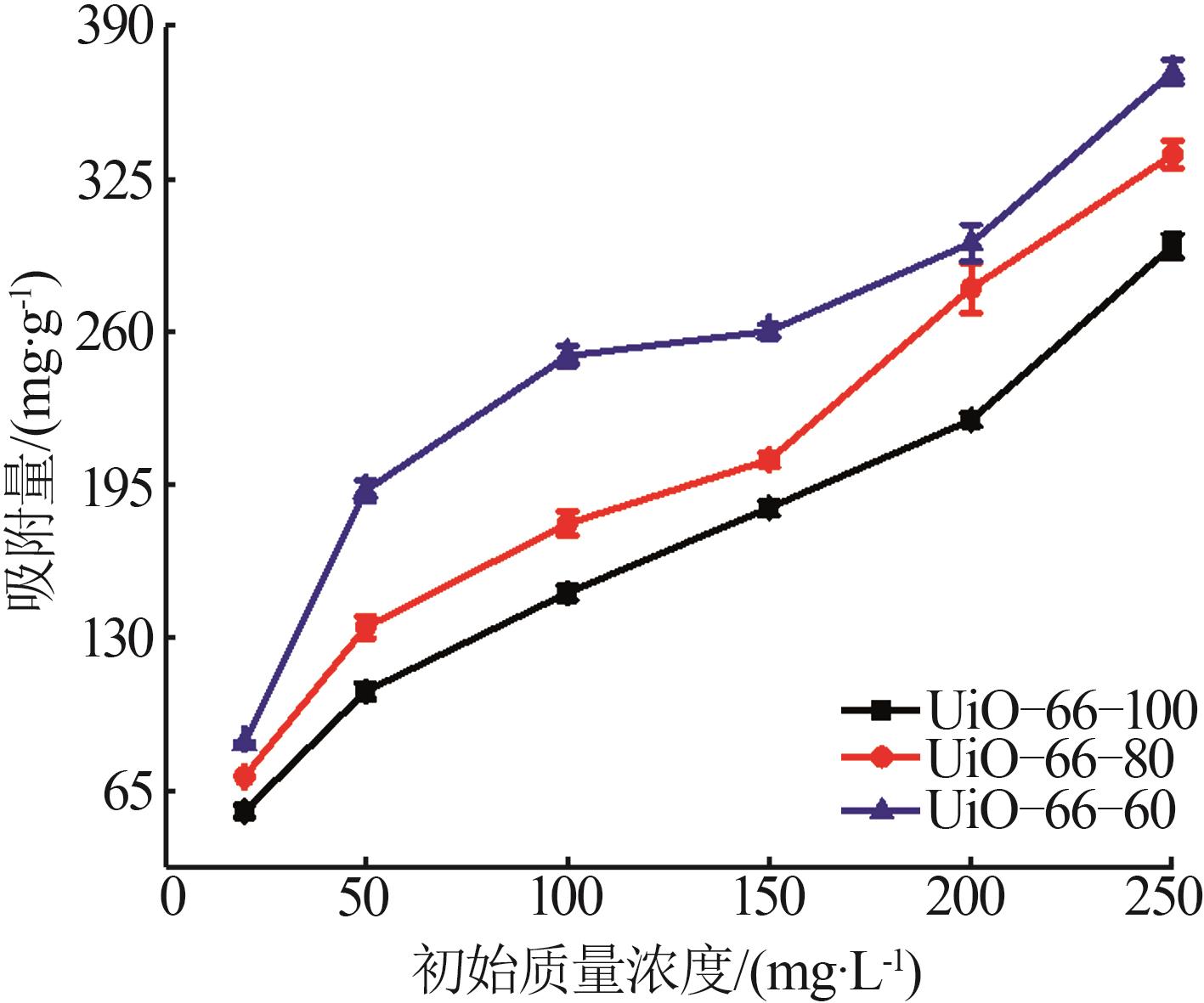
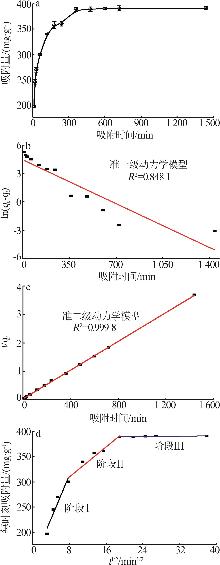
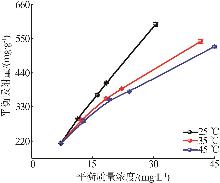
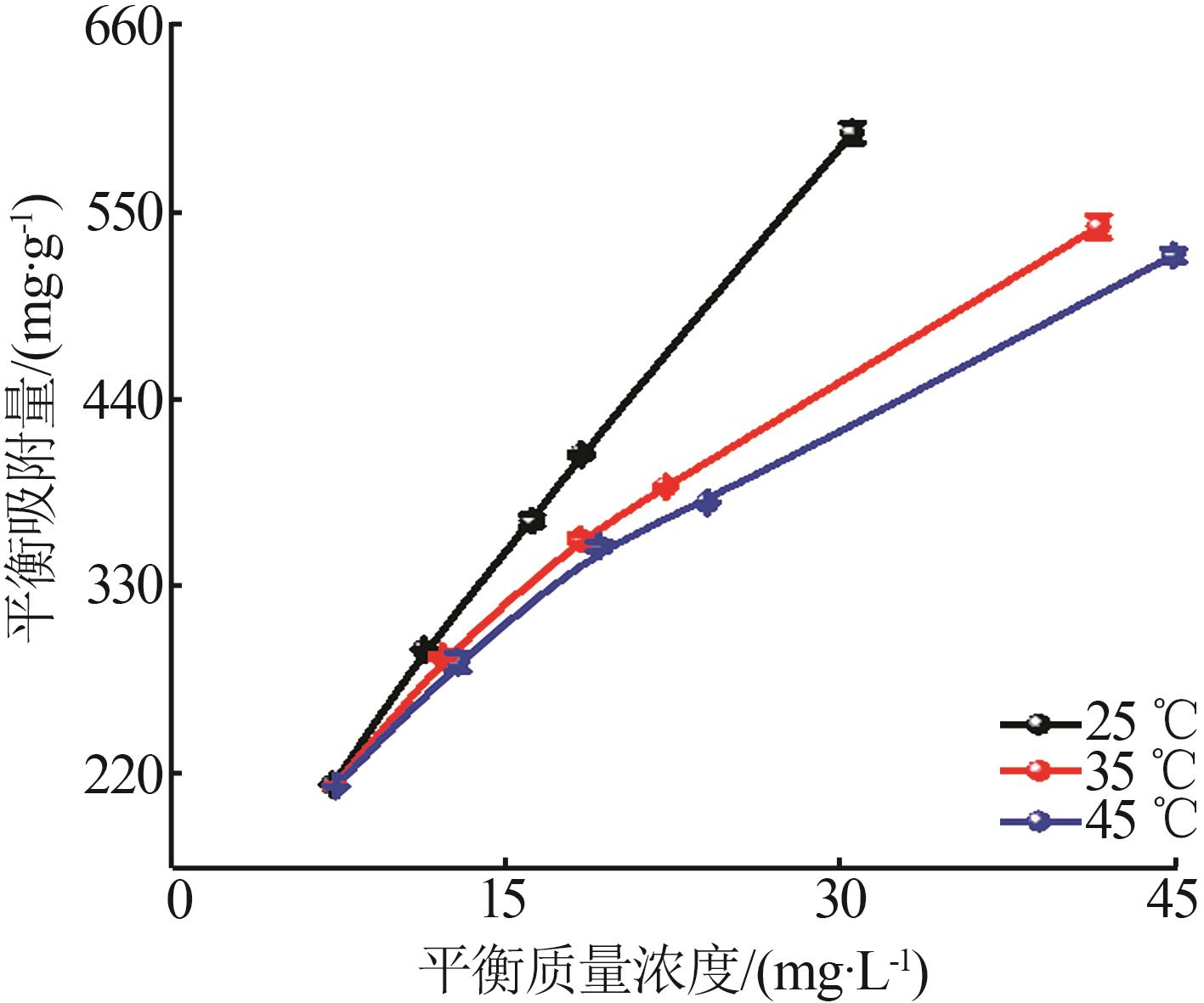
Inorganic Chemicals Industry ›› 2026, Vol. 58 ›› Issue (1): 26-36.doi: 10.19964/j.issn.1006-4990.2025-0108
• Research & Development • Previous Articles Next Articles
Study on low⁃temperature synthesis of UiO-66 and its adsorption performance for removing methyl orange from water
LIU Yixian( ), MA Xilong, FANG Chunxia, XIE Juan, ZHANG Zhikun, LI Pengfei, LI Zhengjie(
), MA Xilong, FANG Chunxia, XIE Juan, ZHANG Zhikun, LI Pengfei, LI Zhengjie( ), HAN Jilong(
), HAN Jilong( )
)
- School of Chemical and Pharmaceutical Engineering,Hebei University of Science and Technology,Shijiazhuang 050018
-
Received:2025-03-05Online:2026-01-10Published:2025-06-30 -
Contact:LI Zhengjie, HAN Jilong E-mail:871090182@qq.com;lizj@hebust.edu.cn;hanjilong@hebust.edu.cn
CLC Number:
Cite this article
LIU Yixian, MA Xilong, FANG Chunxia, XIE Juan, ZHANG Zhikun, LI Pengfei, LI Zhengjie, HAN Jilong. Study on low⁃temperature synthesis of UiO-66 and its adsorption performance for removing methyl orange from water[J]. Inorganic Chemicals Industry, 2026, 58(1): 26-36.
share this article
Table 4
Comparison of adsorption capacity of MO between UiO-66-60 and other adsorbents"
| 吸附剂 | qmax/(mg·g-1) | 吸附条件 |
|---|---|---|
| Cu2O/CuO-g-C3N4[ | 241.3 | 添加量0.2 g/L,pH 6.0,30 ℃ |
Fe3O4/MIL101 (Al0.9Fe0.1)/NH2[ | 355.8* | 添加量0.4 g/L,pH 6.0,50 ℃ |
| Cs/UiO-66[ | 370.4* | 添加量0.5 g/L,pH 3.0,30 ℃ |
| MIL-68(Al)[ | 340.1* | 添加量0.4 g/L,pH 8.0,35 ℃ |
| UIO-g-NL[ | 961.5 | 添加量0.4 g/L,pH 5.0,25 ℃ |
| N-NC-800[ | 222.2* | 添加量0.4 g/L,pH 6.0,25 ℃ |
| UiO-66[ | 256.4* | 添加量0.5 g/L,pH 3.0,30 ℃ |
| UiO-66-NH2[ | 148.4 | 添加量0.4 g/L,pH 5.0,25 ℃ |
| Ce-doped UiO-66[ | 639.6 | 添加量0.2 g/L,自然pH,25 ℃ |
| UiO-66-60 | 597.1 | 添加量0.2 g/L,pH 3.0,25 ℃ |
| [1] | 张冲,郭云,彭子芳,等.三聚氰胺功能化多孔有机聚合物的合成及其对甲基橙的吸附性能[J].色谱,2021,39(9):998-1005. |
| ZHANG Chong, GUO Yun, PENG Zifang,et al.Preparation of melamine⁃functionalized porous organic polymer and its adsorption properties for methyl orange[J].Chinese Journal of Chromatography,2021,39(9):998-1005. | |
| [2] | UGRASKAN V, ISIK B, YAZICI O,et al.Removal of Safranine T by a highly efficient adsorbent(Cotinus Coggygria Leaves):Isotherms,kinetics,thermodynamics,and surface properties[J].Surfaces and Interfaces,2022,28:101615. |
| [3] | 王萍,徐荣声,孙冬,等.氮掺杂生物炭的制备及其对亚甲基蓝的吸附性能研究[J].无机盐工业,2024,56(9):117-127. |
| WANG Ping, XU Rongsheng, SUN Dong,et al.Study on preparation of nitrogen⁃doped biochar and its adsorption properties for methylene blue[J].Inorganic Chemicals Industry,2024,56(9):117-127. | |
| [4] | SOLAYMAN H M, HOSSEN M A, AZIZ A ABD,et al.Performance evaluation of dye wastewater treatment technologies:A review[J].Journal of Environmental Chemical Engineering,2023,11(3):109610. |
| [5] | 张涵飞,申红艳,刘有智.MG复合材料的制备及其对甲基橙的吸附性能研究[J].无机盐工业,2025,57(6):100-107. |
| ZHANG Hanfei, SHEN Hongyan, LIU Youzhi.Study on preparation of MG composite material and its adsorption properties for methyl orange[J].Inorganic Chemicals Industry,2025,57(6):100-107. | |
| [6] | KAYANI K F.Bimetallic metal-organic frameworks (BMOFs) for dye removal:A review[J].RSC Advances,2024,14(43):31777-31796. |
| [7] | 裴秀,李亚明.共价有机框架材料的制备及对染料吸附性能的研究[J].无机盐工业,2023,55(1):106-111. |
| PEI Xiu, LI Yaming.Study on preparation of covalent organic framework materials and their adsorption properties for dyes[J].Inorganic Chemicals Industry,2023,55(1):106-111. | |
| [8] | 赵英杰,赵慧芳,王婷,等.金属-有机骨架材料用于水中痕量药物污染物的吸附脱除[J].化工进展,2020,39(6):2187-2205. |
| ZHAO Yingjie, ZHAO Huifang, WANG Ting,et al.Adsorption removal of trace pharmaceutical pollutants from water by metal-organic frameworks[J].Chemical Industry and Engineering Progress,2020,39(6):2187-2205. | |
| [9] | MANDAL W, FAJAL S, DESAI A V,et al.Metal-organic frameworks(MOFs) and related other advanced porous materials for sequestration of heavy metal⁃based toxic oxo⁃pollutants from water[J].Coordination Chemistry Reviews,2025,524:216326. |
| [10] | 韩慧敏,袁静珂,何柏,等.UiO-66的合成、结构及应用进展[J].精细化工,2023,40(6):1187-1201,1238. |
| HAN Huimin, YUAN Jingke, HE Bai,et al.Progress on synthesis,structure and application of UiO-66[J].Fine Chemicals,2023,40(6):1187-1201,1238. | |
| [11] | 张静,刘洁.UiO系列金属有机骨架的合成方法及其吸附应用[J].功能材料,2022,53(10):10087-10094. |
| ZHANG Jing, LIU Jie.Synthetic methods of UiO series metal-organic frameworks and their applications in adsorption[J].Journal of Functional Materials,2022,53(10):10087-10094. | |
| [12] | HASAN Z, KHAN N A, JHUNG S H.Adsorptive removal of diclofenac sodium from water with Zr⁃based metal-organic frameworks[J].Chemical Engineering Journal,2016,284:1406-1413. |
| [13] | WANG Chenghong, LIU Xinlei, CHEN J P,et al.Superior removal of arsenic from water with zirconium metal-organic framework UiO-66[J].Scientific Reports,2015,5:16613. |
| [14] | AHMADIJOKANI F, MOLAVI H, REZAKAZEMI M,et al.UiO⁃66 metal-organic frameworks in water treatment:A critical review[J].Progress in Materials Science,2022,125:100904. |
| [15] | ŁUCZAK J, KROCZEWSKA M, BALUK M,et al.Morphology control through the synthesis of metal-organic frameworks[J].Advances in Colloid and Interface Science,2023,314:102864. |
| [16] | ALAERTS L, SÉGUIN E, POELMAN H,et al.Probing the lewis acidity and catalytic activity of the metal⁃organic framework[Cu3(btc)2] (BTC=Benzene-1,3,5-tricarboxylate)[J].Chemistry:A European Journal,2006,12(28):7353-7363. |
| [17] | JAJKO G, GUTIÉRREZ-SEVILLANO J J, SŁAWEK A,et al.Water adsorption in ideal and defective UiO-66 structures[J].Microporous and Mesoporous Materials,2022,330:111555. |
| [18] | DESTEFANO M R, ISLAMOGLU T, GARIBAY S J,et al.Room⁃temperature synthesis of UiO-66 and thermal modulation of densities of defect sites[J].Chemistry of Materials,2017,29(3):1357-1361. |
| [19] | JAJKO G, GRYTA P, KOZYRA P,et al.Effect of synthesis temperature on water adsorption in UiO-66 derivatives:Experiment,DFT+D modeling,and Monte Carlo simulations[J].The Journal of Physical Chemistry C,2022,126(21):9185-9194. |
| [20] | KIM J Y, KANG J,CHA S,et al.Stability of Zr⁃based UiO-66 metal-organic frameworks in basic solutions[J].Nanomaterials,2024,14(1):110. |
| [21] | AHMADIJOKANI F, MOHAMMADKHANI R, AHMADIPOUYA S,et al.Superior chemical stability of UiO-66 metal-organic fra⁃meworks(MOFs) for selective dye adsorption[J].Chemical Engineering Journal,2020,399:125346. |
| [22] | WAN Dongjin, CHENG Xiaofan, SHI Yahui,et al.Insights into lead removal in water using a novel carbonized material derived from the by⁃product of oil refining:Action mechanism and performance optimization[J].Journal of Chemical Technology & Biotechnology,2021,96(11):3224-3236. |
| [23] | LAFI R, HAFIANE A.Removal of methyl orange (MO) from aqueous solution using cationic surfactants modified coffee waste(MCWs)[J].Journal of the Taiwan Institute of Chemical Engineers,2016,58:424-433. |
| [24] | 熊波,黎泰华,周武平,等.一步热聚合法制备Cu2O/CuO-g-C3N4吸附剂及其对甲基橙吸附的性能[J].应用化学,2023,40(3):420-429. |
| XIONG Bo, LI Taihua, ZHOU Wuping,et al.Preparation of Cu2O/CuO-g-C3N4 adsorbent by one⁃step thermal polymerization and adsorption properties for methyl orange[J].Chinese Journal of Applied Chemistry,2023,40(3):420-429. | |
| [25] | NAGA A O ABO EL, SHABAN S A, KADY F Y A EL.Metal organic framework⁃derived nitrogen⁃doped nanoporous carbon as an efficient adsorbent for methyl orange removal from aqueous solution[J].Journal of the Taiwan Institute of Chemical Engineers,2018,93:363-373. |
| [26] | BAO Shuangyou, LI Kai, NING Ping,et al.Synthesis of amino⁃functionalization magnetic multi⁃metal organic framework(Fe3O4/MIL-101(Al0.9Fe0.1)/NH2) for efficient removal of methyl orange from aqueous solution[J].Journal of the Taiwan Institute of Chemical Engineers,2018,87:64-72. |
| [27] | EDIATI R, AULIA W, NIKMATIN B A,et al.Chitosan/UiO-66 composites as high⁃performance adsorbents for the removal of methyl orange in aqueous solution[J].Materials Today Chemistry,2021,21:100533. |
| [28] | LV Shiwen, LIU Jingmin, MA Hui,et al.Simultaneous adsorption of methyl orange and methylene blue from aqueous solution using amino functionalized Zr⁃based MOFs[J].Microporous and Mesoporous Materials,2019,282:179-187. |
| [29] | WU Shichuan, YOU Xia, YANG Cao,et al.Adsorption behavior of methyl orange onto an aluminum⁃based metal organic framework,MIL-68(Al)[J].Water Science and Technology,2017,75(12):2800-2810. |
| [30] | YANG Jimin, YING Rongjian, HAN Chunxiang,et al.Adsorptive removal of organic dyes from aqueous solution by a Zr⁃based metal⁃organic framework:Effects of Ce(Ⅲ) doping[J].Dalton Transactions,2018,47(11):3913-3920. |
| [31] | WANG Chao, FENG Xuezhen, SHANG Shibin,et al.Adsorption of methyl orange from aqueous solution with lignin⁃modified metal-organic frameworks:Selective adsorption and high adsorption capacity[J].Bioresource Technology,2023,388:129781. |
| [32] | 王伟涛,陈香李,杨百勤.固体在液相中吸附热力学参数计算介绍[J].大学化学,2021,36(2):233-240. |
| WANG Weitao, CHEN Xiangli, YANG Baiqin.Calculation of adsorption thermodynamics parameters for adsorption on the solid⁃liquid interface[J].University Chemistry,2021,36(2):233-240. |
| [1] | ZHANG Hanfei, SHEN Hongyan, LIU Youzhi. Study on preparation of MG composite material and its adsorption properties for methyl orange [J]. Inorganic Chemicals Industry, 2025, 57(6): 100-107. |
| [2] | HE Yipeng, XIONG Chenxi, WANG Yiping, LI Jun, JIN Yang. Research on preparation of iron-based organic metal-organic framework at room temperature for adsorption of trivalent arsenic [J]. Inorganic Chemicals Industry, 2024, 56(2): 111-120. |
| [3] | WANG Mengdi, LUO Jin, WU Wei, ZHOU Jinghui, WANG Jing, SUN Yanmin, YU Haibin. Study on preparation of γ-Al2O3 by microreaction and its properties for methyl orange adsorption [J]. Inorganic Chemicals Industry, 2023, 55(9): 66-74. |
| [4] | LIU Zihan, XI Guojun, LEI Guangping. Application of MOFs in adsorption refrigeration/heat pump [J]. Inorganic Chemicals Industry, 2023, 55(4): 20-26. |
| [5] | Shen Wei,Wang Sinan,Liang Xuemei,Wei Jinyun,Pan Yujie,Nong Tiantian,Zhou Yan,Tan Xuecai,Huang Zaiyin. Research progress of nano MOFs and their derivatives for supercapacitors [J]. Inorganic Chemicals Industry, 2021, 53(6): 79-86. |
| [6] | Sun Haijie,Liu Xin′gai,Chen Zhihao,Chen Lingxia,Deng Yaru,Mei Yangyang. Study on photocatalytic degradation of methyl orange by BiOI/g-C3N4 [J]. Inorganic Chemicals Industry, 2021, 53(4): 90-94. |
| [7] | Shu Yirui,Zhang Pan,Wang Wei,Xiang Hengli,Ren Genkuan,Xu Dehua,Zhang Zhiye,Yang Xiushan. Titanium white by-product ferrous sulfate photofenton oxidation degradation of methyl orange in wastewater [J]. Inorganic Chemicals Industry, 2021, 53(3): 68-72. |
| [8] | Han Xiaogang,Min Jianjun,Gu Yifei,Liu Zhuannian. Preparation of CaFeAl-LDO from polyaluminum chloride residue and its adsorption for methyl orange [J]. Inorganic Chemicals Industry, 2021, 53(10): 81-85. |
| [9] | Sun Haijie,Shao Bingqi,Chen Lingxia,Cai Wenjuan,Chen Yiyan,Dou Xiaoya,Yang Mengli. Performance of BiOI/SiO2 catalyst for photocatalytic degradation of methyl orange [J]. Inorganic Chemicals Industry, 2019, 51(7): 89-92. |
| [10] | HAO Yan, MA Xue-Lian, GUO Gui-Bao, JIA Yi-Ji, BI Xin, HUANG Jiang-Dong. Study on preparation and photocatalytic properties of nano-sized ZnO supported on activated carbon by hydrothermal method [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(2): 71-. |
| [11] | SUN Huai-Yu, FANG Wei, LI Xue. Preparation and photocatalytic performance of Pb-TiO2 films [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(9): 75-. |
| [12] | LIU Xi-Xiang, 欧Yang-Hui-Xiang , XIE Yu-Qi, LING Shao-Ming, LAN Cui-Ling. Mimic enzyme catalytic spectrophotometric determination of trace hydrogen peroxide in foodstuff with Fe3O4 nanoparticles [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(5): 66-. |
| [13] | YUAN Chun-Hua, XIE Ying-Na. Preparation of cobalt-doped titania photocatalysts and photocatalytic activity thereof [J]. INORGANICCHEMICALSINDUSTRY, 2011, 43(11): 31-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||