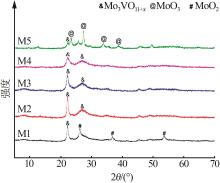
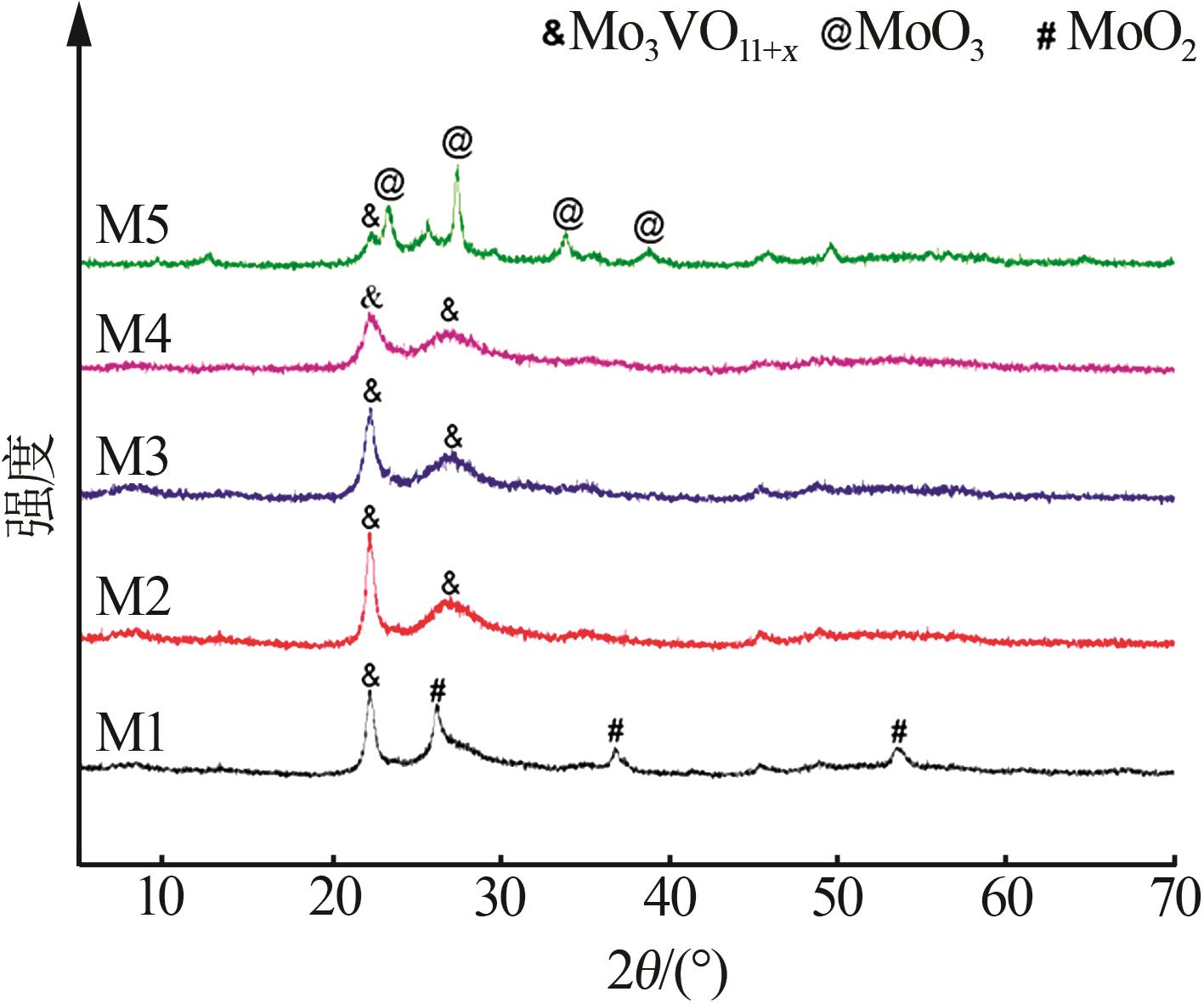
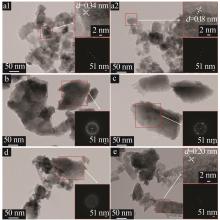
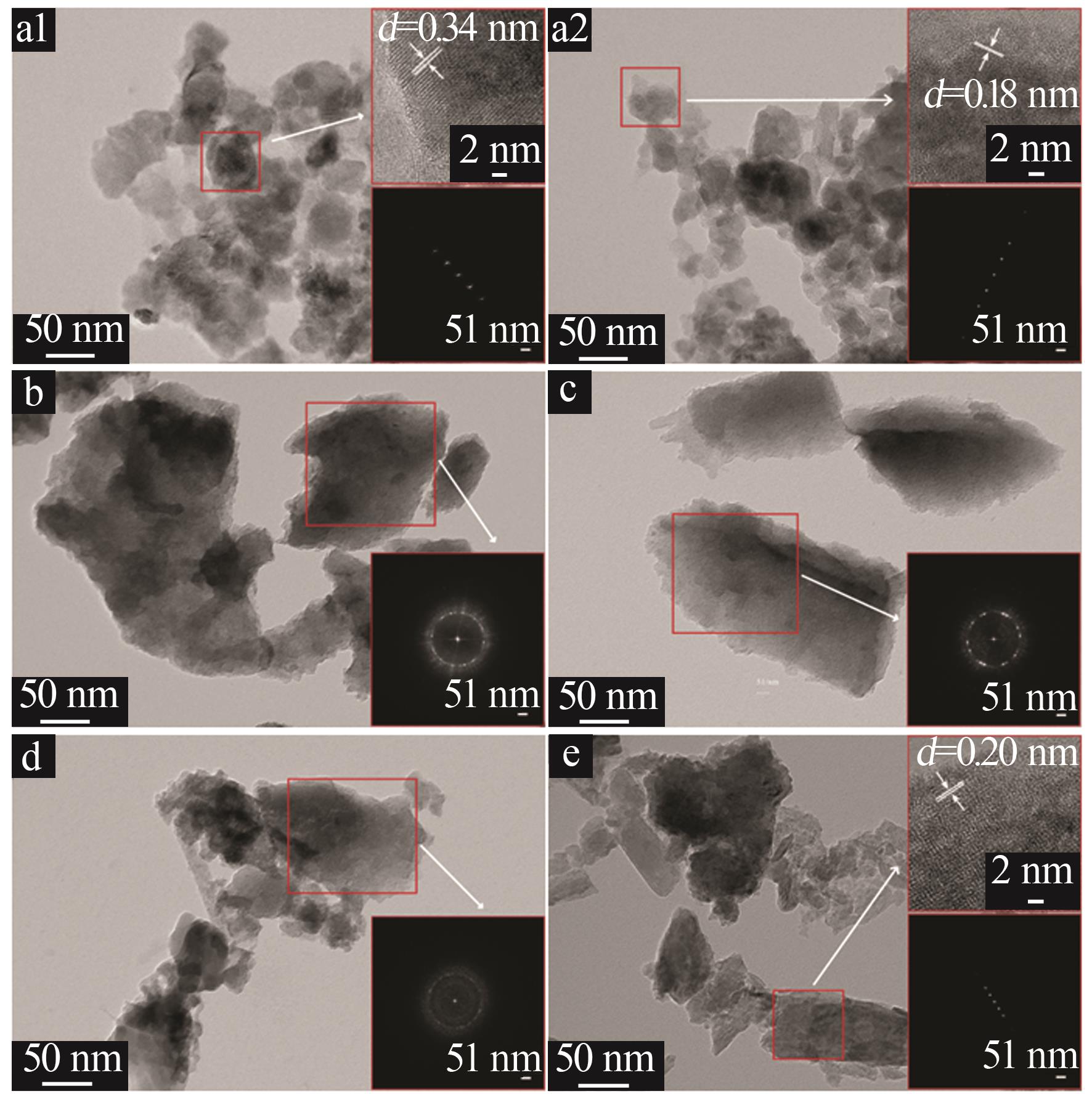
| 1 |
ANDRUSHKEVICH T V.Heterogeneous catalytic oxidation of acrolein to acrylic acid:Mechanism and catalysts[J].Catalysis Reviews,1993,35(2):213-259.
|
| 2 |
TICHÝ J.Oxidation of acrolein to acrylic acid over vanadium-molybdenum oxide catalysts[J].Applied Catalysis A:General,1997,157(1/2):363-385.
|
| 3 |
FANTIM W M, ROCHA POÇO J G, DERENZO S.Synthesis of catalysts containing mixed oxides of Mo-V-Cu-W to produce acrylic acid:A comparison between hydrothermal synthesis and coprecipitation[J].Chemical Engineering & Technology,2024,47(1):117-127.
|
| 4 |
CHEN Chen, KOSUKE N, MURAYAMA T,et al.Single-crystalli-ne-phase Mo3VO x :An efficient catalyst for the partial oxidation of acrolein to acrylic acid[J].ChemCatChem,2013,5(10):2869-2873.
|
| 5 |
PANOV G I, STAROKON E V, PARFENOV M V,et al.Quasi-catalytic identification of intermediates in the oxidation of propene to acrolein over a multicomponent Bi-Mo catalyst[J].ACS Catalysis,2018,8(2):1173-1177.
|
| 6 |
BELL A T.Insights into the mechanism and kinetics of propene oxidation and ammoxidation over bismuth molybdate catalysts derived from experiments and theory[J].Journal of Catalysis,2022,408:436-452.
|
| 7 |
KNOCHE S, HEID M, GORA N,et al.Mechanistic study on the selective oxidation of acrolein to acrylic acid concerning the role of water[J].ChemCatChem,2020,12(13):3560-3575.
|
| 8 |
KNOBL S.The synthesis and structure of a single-phase,nanocrystalline MoVW mixed-oxide catalyst of the Mo5O14 type[J].Journal of Catalysis,2003,215(2):177-187.
|
| 9 |
OVSITSER O, UCHIDA Y, MESTL G,et al.Molybdenum oxide based partial oxidation catalyst[J].Journal of Molecular Catalysis A:Chemical,2002,185(1/2):291-303.
|
| 10 |
WANG Weihua, XU Wenjie, SONG Weilin,et al.Novel Mo-V oxide catalysts with nanospheres as templates for the selective oxidation of acrolein to acrylic acid[J].Catalysis Letters,2021,151(8):2326-2338.
|
| 11 |
ISHIKAWA S, YAMADA Y, KASHIO N,et al.True catalytically active structure in Mo-V-based mixed oxide catalysts for selective oxidation of acrolein[J].ACS Catalysis,2021,11(16):10294-10307.
|
| 12 |
VIEIRA L H, MARTINS L, POSSATO L G.Fundamentals and development of oxidation catalysts:The acrylic acid manufacture case[J].Catalysis Reviews,2024:1-38.Doi:10.1080/01614940.2024 .
|
| 13 |
WU Shutao, SHE Qiming, TESSER R,et al.Catalytic glycerol dehydration-oxidation to acrylic acid[J].Catalysis Reviews,2020,62(4):481-523.
|
| 14 |
LUMONGGA PUTRI TAMBUNAN M, ABDULLAH I, KRISYUNINGSIH KRISNANDI Y.One-pot glycerol conversion to acrylic acid catalyzed by Cu modified HY zeolite synthesized from natural resources and pro-analytical precursor[J].Carbon Resources Conversion,2024,7(1):100188.
|
| 15 |
AHMAD M Y, BASIR N I, ABDULLAH A Z.A review on one-pot synthesis of acrylic acid from glycerol on bi-functional catalys-ts[J].Journal of Industrial and Engineering Chemistry,2021,93:216-227.
|
| 16 |
DIETERLE M, MESTL G, JÄGER J,et al.Mixed molybdenum oxide based partial oxidation catalyst[J].Journal of Molecular Catalysis A:Chemical,2001,174(1/2):169-185.
|
| 17 |
VÉDRINE J C.Metal oxides in heterogeneous catalysis[M].Elsevier,2018,551-569.
|
| 18 |
H·希斯特,A·谭坦,L·马洛斯.多金属氧化物:中国,01124474.7[P].2003-02-05.
|
| 19 |
曾贤君,孙彦民,李晓云,等.Mo-V-O系丙烯酸催化剂表面结构研究[J].工业催化,2013,21(12):40-43.
|
|
ZENG Xianjun, SUN Yanmin, LI Xiaoyun,et al.Research on the surface structure of Mo-V-O acrylic acid catalyst[J].Industrial Catalysis,2013,21(12):40-43.
|
| 20 |
ISHIKAWA S, MURAYAMA T, KUMAKI M,et al.Synthesis of trigonal Mo-V-M3rd-O(M3rd=Fe,W) catalysts by using structure-directing agent and catalytic performances for selective oxidation of ethane[J].Topics in Catalysis,2016,59(17):1477-1488.
|
| 21 |
ENDRES S, KAMPE P, KUNERT J,et al.The influence of tungsten on structure and activity of Mo-V-W-mixed oxide catalysts for acrolein oxidation[J].Applied Catalysis A:General,2007,325(2):237-243.
|
| 22 |
DONG Wei, ZHOU Shian, MA Yan,et al.N-doped C-coated MoO2/ZnIn2S4 heterojunction for efficient photocatalytic hydrogen production[J].Rare Metals,2023,42(4):1195-1204.
|
| 23 |
CHEN Jing, CHEN Xuanle, LU Rou,et al.Necklace-like carbon nanofibers encapsulating MoO2 nanospheres with Mo-C bonding for stable lithium-ion storage[J].Rare Metals,2023,42(8):2592-2600.
|
| 24 |
LIU Jun, TANG Shasha, LU Yakun,et al.Synthesis of Mo2N nanolayer coated MoO2 hollow nanostructures as high-performance anode materials for lithium-ion batteries[J].Energy & Environmental Science,2013,6(9):2691-2697.
|
| 25 |
祁琰媛,陈文,麦立强,等.水热合成MoO3纳米带的生长机理研究[J].无机化学学报,2007,23(11):1895-1900.
|
|
QI Yanyuan, CHEN Wen, Liqiang MAI,et al.Growth mechanism of MoO3 nanobelts under hydrothermal condition[J].Chinese Journal of Inorganic Chemistry,2007,23(11):1895-1900.
|
| 26 |
KOLTUNOV K Y, SOBOLEV V I, BONDAREVA V M.Oxidation,oxidative esterification and ammoxidation of acrolein over metal oxides:Do these reactions include nucleophilic acyl substitution?[J].Catalysis Today,2017,279:90-94.
|
| 27 |
王红心,邓忠华,楚文玲,等.丙烷氧化制丙烯酸催化剂MoVTeNbO的两段焙烧[J].催化学报,2009,30(6):490-496.
|
|
WANG Hongxin, DENG Zhonghua, CHU Wenling,et al.Two-step calcined MoVTeNbO catalysts for the oxidation of propane to acrylic acid[J].Chinese Journal of Catalysis,2009,30(6):490-496.
|
| 28 |
李双明,石倩翡,徐雷雷,等.高比表面介孔MoVTeNbO催化剂的制备及其催化性能[J].分子催化,2015,29(1):9-18.
|
|
LI Shuangming, SHI Qianfei, XU Leilei,et al.Preparation of high specific surface mesoporous MoVTeNbO catalyst and its catalytic properties[J].Journal of Molecular Catalysis(China),2015,29(1):9-18.
|
| 29 |
KAMPE P, GIEBELER L, SAMUELIS D,et al.Heterogeneously catalysed partial oxidation of acrolein to acrylic acid:Structure,function and dynamics of the V-Mo-W mixed oxides[J].Physical Chemistry Chemical Physics:PCCP,2007,9(27):3577-3589.
|
 ), SU Jin, ZHANG Lijie, ZHOU Peng, SUN Yanmin, LI Jia
), SU Jin, ZHANG Lijie, ZHOU Peng, SUN Yanmin, LI Jia