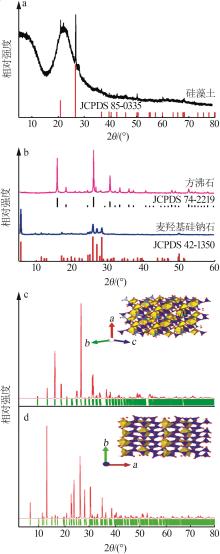
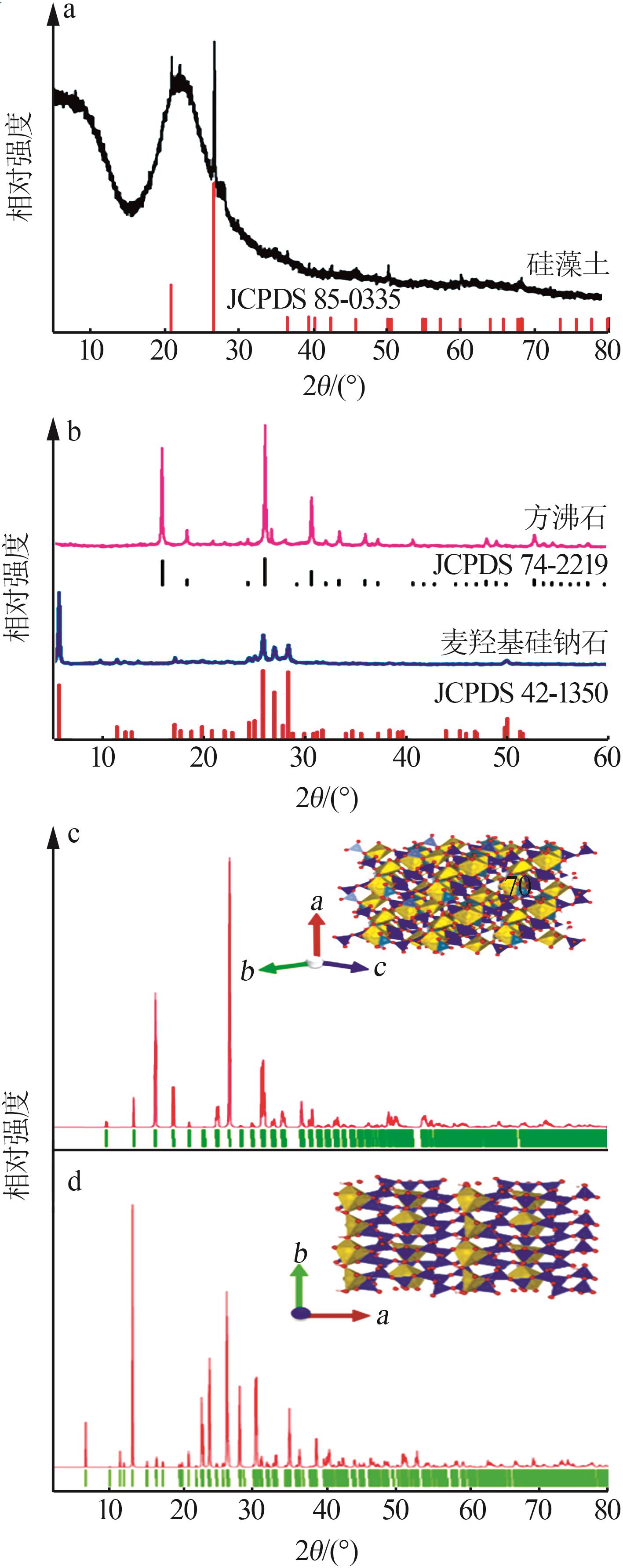
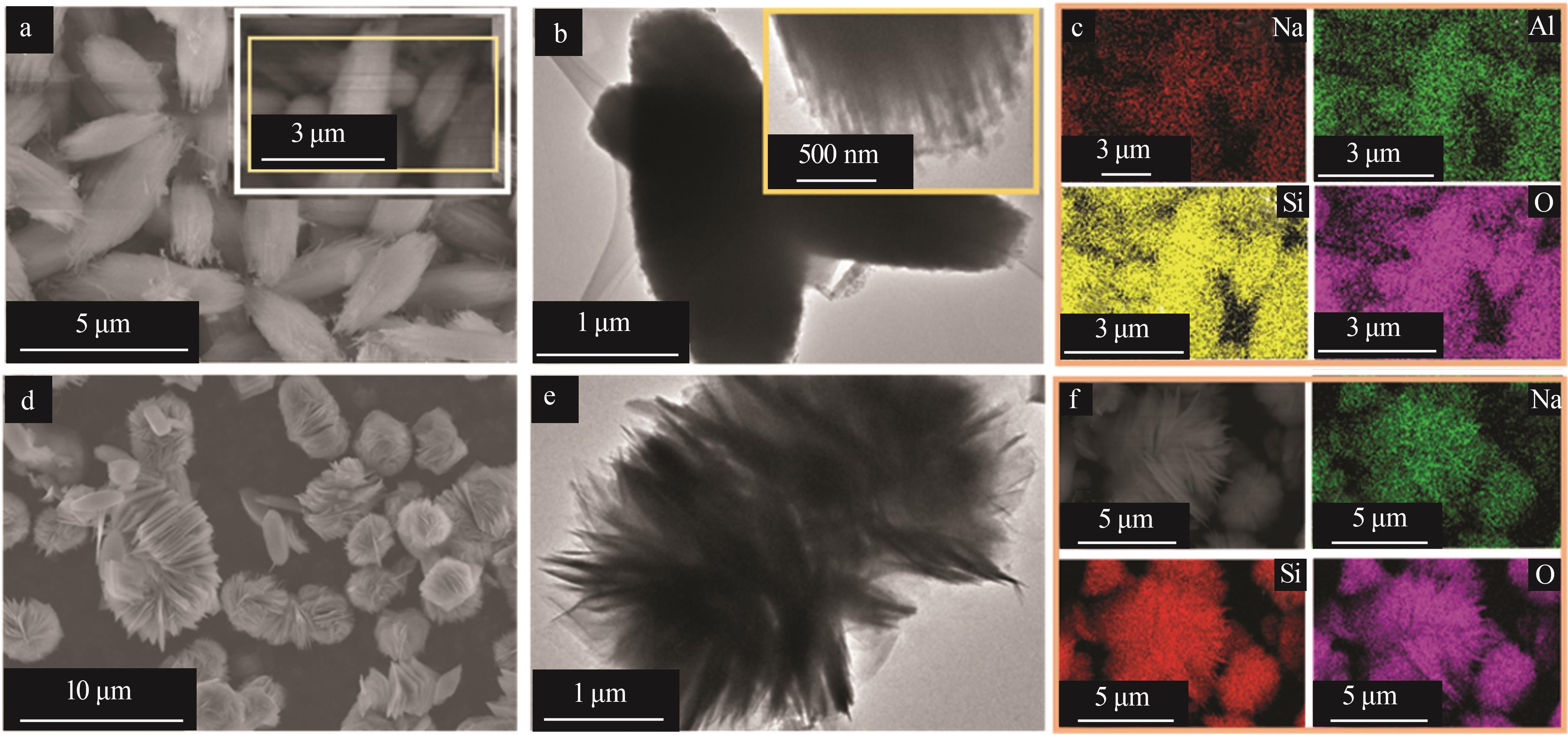
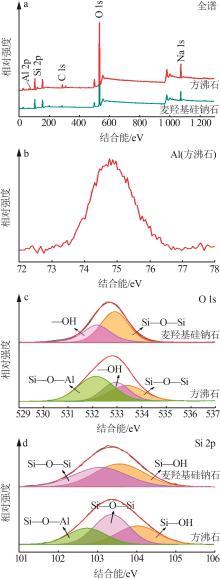
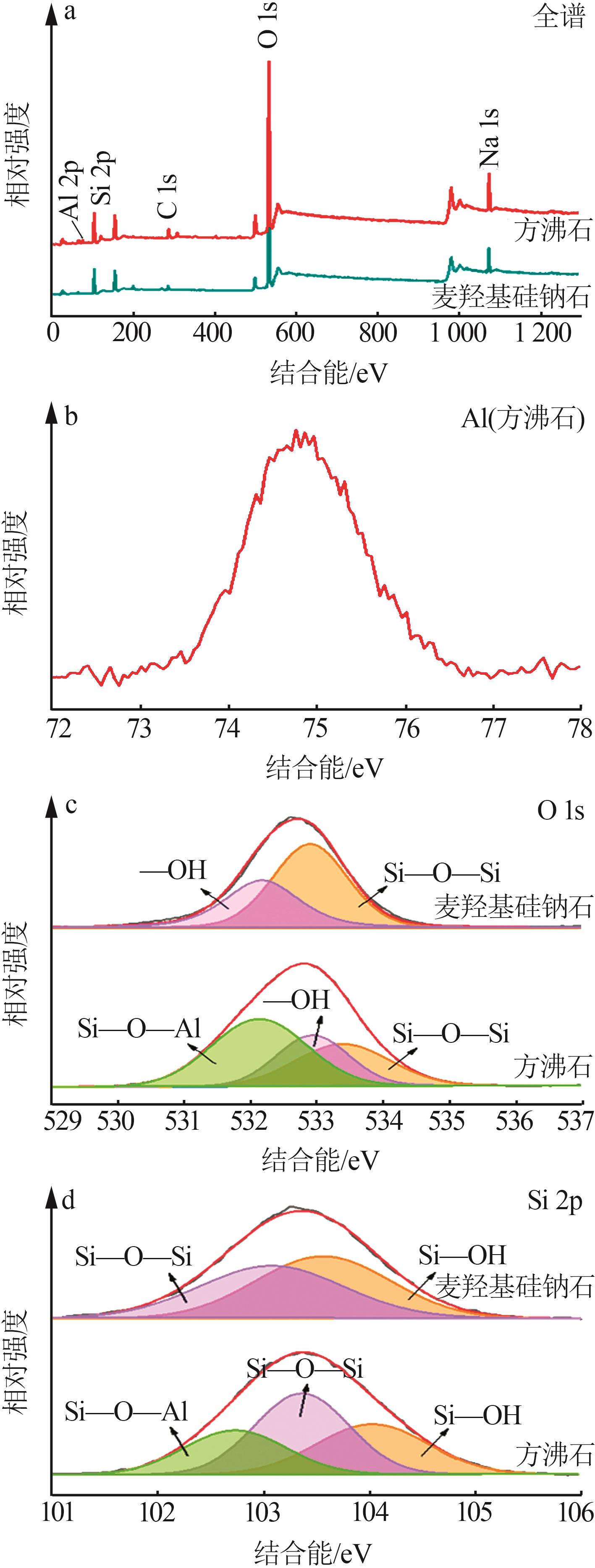
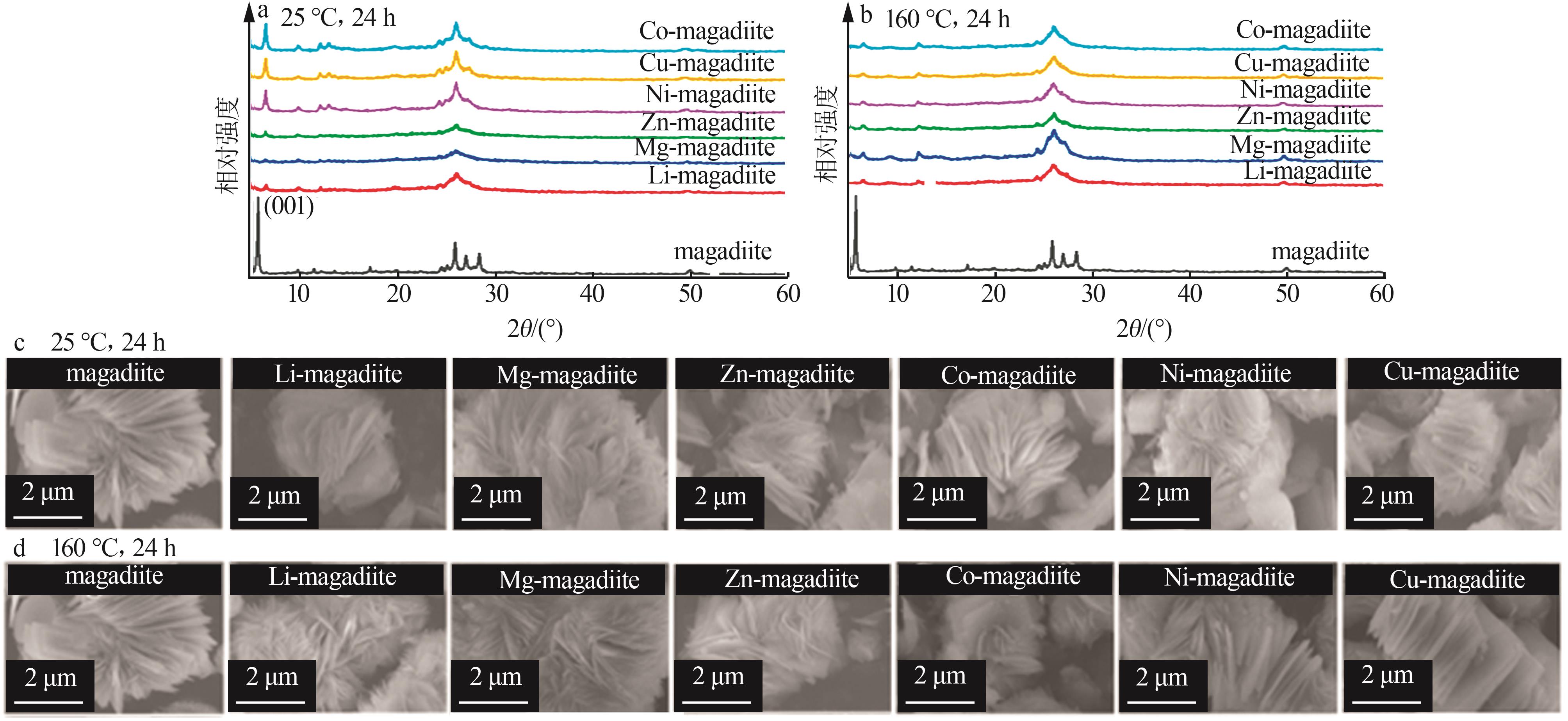
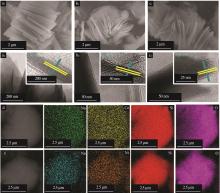
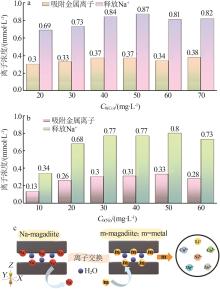
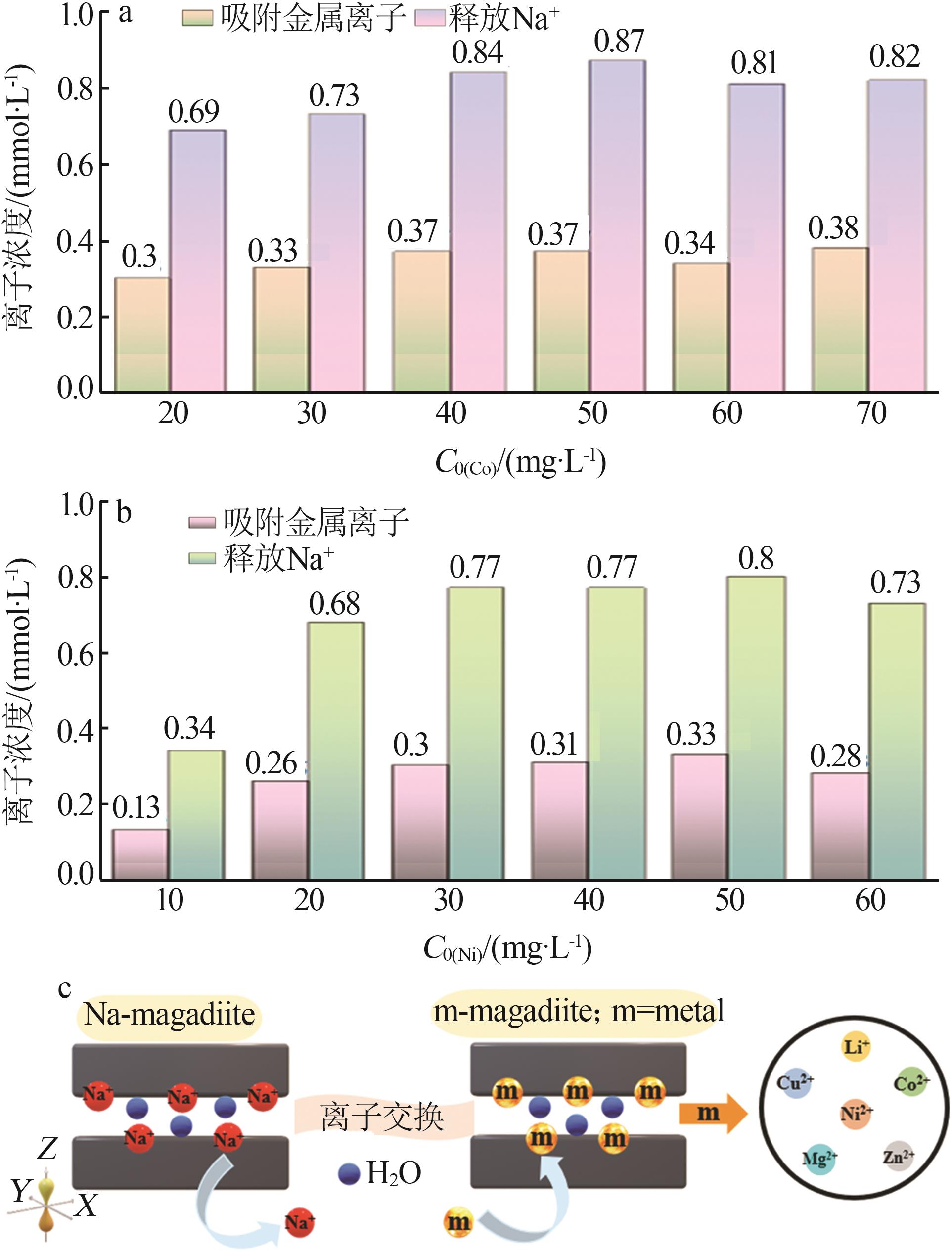
Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (2): 45-54.doi: 10.19964/j.issn.1006-4990.2022-0537
• Research & Development • Previous Articles Next Articles
Study on controlled synthesis of magadiite and its metal ion exchange properties
GUO Zhanzhan1( ),ZHOU Wenyuan1,YANG Yunfei1,WU Junshu1(
),ZHOU Wenyuan1,YANG Yunfei1,WU Junshu1( ),WANG Jinshu1,SUN Lingmin1,ZHANG Zhigang2,DU Yucheng1
),WANG Jinshu1,SUN Lingmin1,ZHANG Zhigang2,DU Yucheng1
- 1. Beijing University of Technology,Beijing 100124,China
2. China ENFI Engineering Co. ,Ltd. ,Beijing 100038,China
-
Received:2022-09-06Online:2023-02-10Published:2023-02-16 -
Contact:WU Junshu E-mail:2550799438@qq.com;junshuwu@bjut.edu.cn
CLC Number:
Cite this article
GUO Zhanzhan,ZHOU Wenyuan,YANG Yunfei,WU Junshu,WANG Jinshu,SUN Lingmin,ZHANG Zhigang,DU Yucheng. Study on controlled synthesis of magadiite and its metal ion exchange properties[J]. Inorganic Chemicals Industry, 2023, 55(2): 45-54.
share this article

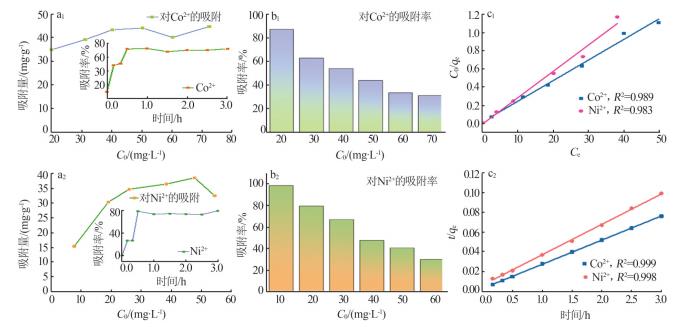
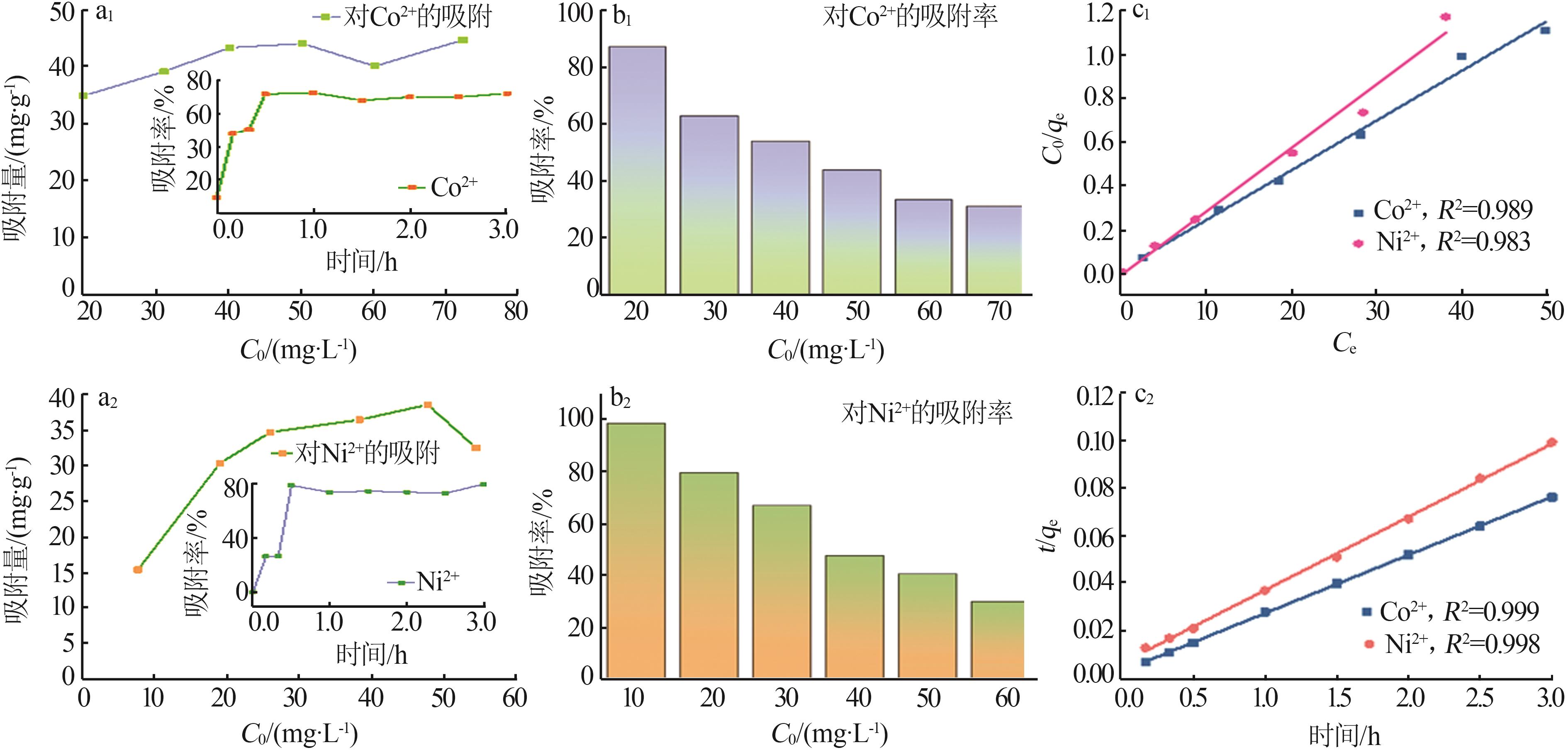
Fig.7
Equilibrium adsorption capacity as a function of initial concentration C0 of Co2+ and Ni2+ (a1,a2),insets are change of adsorption efficiency towards time;Change of adsorption rate as a function of initial concentration C0 of Co2+ and Ni2+ (b1,b2);Langmuir adsorption isotherms of Co2+ and Ni2+(c1);Pseudo second order model fitting of Co2+ and Ni2+ adsorption data(c2)"
| 1 |
LI Jiahao, XIA Chenggong, CHENG Rong,et al.Passivation of multiple heavy metals in lead-zinc tailings facilitated by straw biochar-loaded N-doped carbon aerogel nanoparticles:Mechanisms and microbial community evolution[J].Science of the Total Environment,2022,803.Doi:10.1016/j.scitotenv.2021.149866 .
doi: 10.1016/j.scitotenv.2021.149866 |
| 2 |
HUANG Benqing, TANG Yongjian, GAO Anren,et al.Dually charged polyamide nanofiltration membranes fabricated by micro-wave-assisted grafting for heavy metals removal[J].Journal of Mem-Science brane,2021,640.Doi:10.1016/j.memsci.2021.119834 .
doi: 10.1016/j.memsci.2021.119834 |
| 3 |
CHEN Mingyue, NONG Shuying, ZHAO Yantao,et al.Renewable P-type zeolite for superior absorption of heavy metals:Isotherms,kinetics,and mechanism[J].Science of the Total Environment,2020,726.Doi:10.1016/j.scitotenv.2020.138535 .
doi: 10.1016/j.scitotenv.2020.138535 |
| 4 |
TAN Daoyong, YUAN Peng, ANNABI-BERGAYA F,et al.Methoxy-modified kaolinite as a novel carrier for high-capacity loading and controlled-release of the herbicide amitrole[J].Scientific Reports,2015,5.Doi:10.1038/srep08870 .
doi: 10.1038/srep08870 |
| 5 | 蒋芳,雷婷,李声剑,等.聚合物吸附剂的制备及在水体重金属污染净化应用中的研究进展[J].材料导报,2019,33(S2):526-532. |
| JIANG Fang, LEI Ting, LI Shengjian,et al.Research progress in preparation of polymer adsorbents and purification of heavy metal pollution in water body[J].Materials Reports,2019,33(S2):526-532. | |
| 6 | ARAGAW T A, ALENE A N.A comparative study of acidic,basic,and reactive dyes adsorption from aqueous solution onto Kaolin adsorbent:Effect of operating parameters,isotherms,kinetics,and thermodynamics[J].Emerging Contaminants,2022,8:59-74. |
| 7 | KRISHNA R, VAN BATEN J M.Highlighting the anti-synergy between adsorption and diffusion in cation-exchanged faujasite zeolites[J].ACS Omega,2022,7(15):13050-13056. |
| 8 |
FRANÇA D B, OLIVEIRA L S, NUNES FILHO F G,et al.The versatility of montmorillonite in water remediation using adsorption:Current studies and challenges in drug removal[J].Journal of Environmental Chemical Engineering,2022,10(2).Doi:10.1016/j.jece.2022.107341 .
doi: 10.1016/j.jece.2022.107341 |
| 9 | KWON O Y, JEONG S Y, JEONG K S,et al.Hydrothermal syntheses of Na-magadiite and Na-kenyaite in the presence of carbon-ate[J].Bulletin of the Korean Chemical Society,1995,16(8):737-741. |
| 10 | 彭淑鸽,高秋明.新型纳米层状硅酸盐Magadiite主体材料的制备、表征、结构和生成机理研究[J].高等学校化学学报,2004,25(4):603-606,3. |
| PENG Shuge, GAO Qiuming.Preparation,characterization,structure and formation mechanism of a novel layered structural nanosilicate magadiite host[J].Chemical Research in Chinese Universities,2004,25(4):603-606,3. | |
| 11 | ALMOND G G, HARRIS R K, FRANKLIN K R.A structural consideration of kanemite,octosilicate,magadiite and kenyaite[J].Journal of Materials Chemistry,1997,7(4):681-687. |
| 12 | KOOLI F, MIANHUI L, ALSHAHATEET S F,et al.Characterization and thermal stability properties of intercalated Na-magadiite with cetyltrimethylammonium(C16TMA)surfactants[J].Journal of Physics and Chemistry of Solids,2006,67(5/6):926-931. |
| 13 | WANG Zhen, PINNAVAIA T J.Intercalation of poly(propyleneoxide)amines(Jeffamines)in synthetic layered silicas derived from ilerite,magadiite,and kenyaite[J].Journal of Materials Chemistry,2003,13(9):2127-2131. |
| 14 | BOROWSKI M, KOVALEV O, GIES H.Structural characterization of the hydrous layer silicate Na-RUB-18,Na8Si32O64(OH)8·32H2O and derivatives with XPD-,NPD-,and SS NMR experiments[J].Microporous and Mesoporous Materials,2008,107(1/2):71-80. |
| 15 |
DOS SANTOS T G, DE ASSIS G C, SILVA A O S DA,et al.Progress in development of magadiite to produce multifunctional lamellar materials[J].ACS Applied Materials & Interfaces,2022.Doi:10.1021/acsami.1c15785 .
doi: 10.1021/acsami.1c15785 |
| 16 | FATIMA H, SEO J D, KIM J,et al.Adsorption behavior of kenyaite for Cu2+ and Pb2+ [J].Journal of Porous Materials,2022,29(1):111-117. |
| 17 | MORITA M, HORIUCHI Y, MATSUOKA M,et al.Preparation of titanium-containing layered alkali silicates[J].Crystal Growth & Design,2022,22(3):1638-1644. |
| 18 |
JIANG Dandan, LV Siqi, HAN Xue,et al.Design of Gd3+-immoy selective enrichment of phosphopeptides[J].Mikrochimica Acta,2021,188(10).Doi:10.1007/s00604-021-04972-1 .
doi: 10.1007/s00604-021-04972-1 |
| 19 |
YUAN Zhiqing, TAO Weichuan, WANG Zhendong,et al.One-step synthesis of highly dispersed nanosheets of magadiite[J].Applied Clay Science,2019,181.Doi:10.1016/j.clay.2019. 105231 .
doi: 10.1016/j.clay.2019. 105231 |
| 20 |
ATTAR K, DEMEY H, BOUAZZA D,et al.Sorption and desorption studies of Pb(Ⅱ)and Ni(Ⅱ)from aqueous solutions by a new composite based on alginate and magadiite materials[J].Polymers,2019,11(2).Doi:10.3390/polym11020340 .
doi: 10.3390/polym11020340 |
| 21 |
JÚNIOR A J S F, SODRÉ W C, SOARES B E C F,et al.In situ assembling of layered double hydroxide to magadiite layered silicate with enhanced photocatalytic and recycling performance[J].Applied Surface Science,2021,569.Doi:10.1016/j.apsusc.2021. 151007 .
doi: 10.1016/j.apsusc.2021. 151007 |
| 22 | SHI Zhifang, WANG Yu, MENG Changgong,et al.Hydrothermal conversion of magadiite into mordenite in the presence of cyclohexylamine[J].Microporous and Mesoporous Materials,2013,176:155-161. |
| 23 |
KATABATHINI N, MAKSOD I H ABD EL, MOKHTAR M.Cu,Fe and Mn oxides intercalated SiO2 pillared magadiite and ilerite catalysts for NO decomposition[J].Applied Catalysis A:General,2021,616.Doi:10.1016/j.apcata.2021.118100 .
doi: 10.1016/j.apcata.2021.118100 |
| 24 | LIU Haiyan, SHEN Tong, LI Tiesen,et al.Green synthesis of zeolites from a natural aluminosilicate mineral rectorite:Effects of thermal treatment temperature[J].Applied Clay Science,2014,90:53-60. |
| 25 | 杨晨,吴俊书,王金淑,等.硅藻土的可控沸石化及其对铅离子的吸附固定[J].无机盐工业,2022,54(4):128-134. |
| YANG Chen, WU Junshu, WANG Jinshu,et al.Controlled zeolitization of diatomite for the adsorption and its immobilization of Pb(Ⅱ)ions[J].Inorganic Chemicals Industry,2022,54(4):128-134. | |
| 26 | 孙领民,吴俊书,王金淑,等.低品位硅藻土液相转化合成方钠石重金属吸附剂[J].化学研究,2020,31(4):302-311. |
| SUN Lingmin, WU Junshu, WANG Jinshu,et al.Synthesis of sodalite zeolite from low-grade diatomite for heavy metal adsorpti-on[J].Chemical Research,2020,31(4):302-311. | |
| 27 | LI Keyan, LI Min, XUE Dongfeng.Solution-phase electronegativity scale:Insight into the chemical behaviors of metal ions in solution[J].The Journal of Physical Chemistry.A,2012,116(16):4192-4198. |
| 28 |
HACHEMAOUI M, MOKHTAR A, ISMAIL I,et al.M(M:Cu,Co,Cr or Fe)nanoparticles-loaded metal-organic framework MIL-101(Cr)material by sonication process:Catalytic activity and antibacterial properties[J].Microporous and Mesoporous Materials,2021,323.Doi:10.1016/j.micromeso.2021.111244 .
doi: 10.1016/j.micromeso.2021.111244 |
| 29 |
LU Jitao, ZENG Yue, MA Xiaoxue,et al.Cobalt nanoparticles embedded into N-doped carbon from metal organic frameworks as highly active electrocatalyst for oxygen evolution reaction[J].Polymers,2019,11(5).Doi:10.3390/polym11050828 .
doi: 10.3390/polym11050828 |
| 30 |
JING X, ZHANG Y, DONG X,et al.Layered silicate magadiite-derived three-dimensional honeycomb-like cobalt-nickel silicates as excellent cathode for hybrid supercapacitors[J].Materials Today Chemistry,2021,22.Doi:10.1016/j.mtchem.2021. 100550 .
doi: 10.1016/j.mtchem.2021. 100550 |
| 31 |
YANG Tao, LIU yang, LIU Caixia,et al.Nickel silicate core-shell microspheres hybridized with graphene boosting electrochemical performance[J].Chemical Physics Letters,2020,758.Doi:10.1016/j.cplett.2020.137936 .
doi: 10.1016/j.cplett.2020.137936 |
| [1] | DING Ning, ZHANG Jian, PING Qingwei, SHENG Xueru, LI Na. Study on adsorption and release properties of matrine by magnesium-modified diatomite [J]. Inorganic Chemicals Industry, 2023, 55(11): 37-46. |
| [2] | GUO Xueqin, DENG Xiaochuan, ZHU Chaoliang, FU Xin, WANG Ruirui, MA Wanxia, FAN Jie, ZUO Fangtao, QING Binju. Study on preparation of modified diatomite loaded ammonium phosphomolybdate composite adsorbent and its adsorption performance of Cs+ [J]. Inorganic Chemicals Industry, 2023, 55(11): 19-26. |
| [3] | YANG Chen,WU Junshu,WANG Jinshu,SUN Lingmin,XU Meng,DU Yucheng. Controlled zeolitization of diatomite for the adsorption and its immobilization of Pb(Ⅱ) ions [J]. Inorganic Chemicals Industry, 2022, 54(4): 128-134. |
| [4] | Dong Chaochao,Deng Xiaochuan,Wang Bin,Fan Faying,Zhu Chaoliang,Fan Jie,Qing Binju. Preparation of cesium adsorbent from diatomite and its adsorption properties [J]. Inorganic Chemicals Industry, 2021, 53(6): 134-139. |
| [5] | Wu Zhaomin,Yang Lin,Wang Xinlong,Zhuang Haibo,Ye Runzhou,Wang Ye,Li Yaoji. Study on process of dynamic ion-exchange for etching waste acid containing aluminum in electronic industry [J]. Inorganic Chemicals Industry, 2021, 53(2): 61-65. |
| [6] | Li Ruchun,Zheng Shuilin,Sun Zhiming,Li Chunquan. Research on synthesis of flaky tobermorite from diatomite and its mechanism [J]. Inorganic Chemicals Industry, 2021, 53(1): 24-29. |
| [7] | Tang Qinyuan,An Yan,Sun Qi,Guo Xingqiang,Li Qingqing,Guo Bingchen. Amino-functionalized Al-magadiite for Hg2+ adsorption [J]. Inorganic Chemicals Industry, 2020, 52(8): 40-45. |
| [8] | Xu Xinfang,Zhou Xiaoping,Liu Zhengfeng,Li Lei,Chen Hu,Li Changming. Study on causticization and purification of salt lake lithium ore and recovery of fluorine-containing lithium carbonate [J]. Inorganic Chemicals Industry, 2020, 52(7): 62-65. |
| [9] | Yu Xinrui,Gao Feng,Lü Chao,Sheng Yu,Tong Qingsong. Effects of aging temperature on electrochemical properties of LiNi0.5Co0.2Mn0.3O2 cathode material [J]. Inorganic Chemicals Industry, 2019, 51(10): 51-55. |
| [10] | WANG Yu, 吕Tian-Ming , SHI Li, LIU Xiao-Yu, ZHANG Shou-Lei, LIU Hao. Preparation of mordenite from conversion of layered silicate magadiite [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(1): 17-. |
| [11] | HU Zhi-Bo, LI Jia-Wang, ZHENG Shui-Lin, YAN Yang, XU Chun-Hong. Process research on preparation of sodium silicate by purified diatomite [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(3): 19-. |
| [12] | YAO Huang, PENG Jin-Ping, YU Qian, CHENG Xiao-Ling, LIN Tan-Ting, LI Jun. Preparation and phosphorous removal performance of FeOOH coated diatomite [J]. INORGANICCHEMICALSINDUSTRY, 2011, 43(5): 24-. |
| [13] | Wang Lijian;Zhang Dongyang. Study on modification of nano-TiO2 coated on surface of purified diatomite [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(6): 0-0. |
| [14] | Xia Chenglong;Jing Yan;Jia Yongzhong;Sun Jinhe. Organic modification and physicochemical properties of magnesium lithium silicate [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(4): 0-0. |
| [15] | Zhao Jin;Li Xiangjun;Tian Jianhua;Chen Guishan. Orthogonal experiment of diatomite filter-aid used in preparation of titanium dioxide [J]. INORGANICCHEMICALSINDUSTRY, 2008, 0(6): 0-0. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||